User login
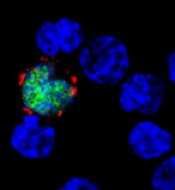
among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()

among uninfected cells (blue)
Image courtesy of
Benjamin Chaigne-Delalande
The European Medicines Agency (EMA) has accepted into its Priority Medicines (PRIME) program an allogeneic cytotoxic T-lymphocyte product that targets Epstein-Barr virus (EBV-CTLs).
EBV-CTLs have been accepted as a treatment for patients with rituximab-refractory EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD).
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About EBV-CTLs
The EBV-CTLs are being developed by Atara Biotherapeutics, Inc.
To create EBV-CTLs, T cells are collected from the blood of third-party donors and exposed to EBV antigens. The activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The EBV-CTLs are designed to find cancer cells expressing EBV and kill them.
Atara Bio’s EBV-CTLs are currently being studied in phase 2 trials of patients with EBV-associated cancers, including PTLD and nasopharyngeal carcinoma.
Results from 2 studies of EBV-CTLs in rituximab-refractory PTLD were presented at the 2015 AACR Annual Meeting.
Atara Bio’s EBV-CTLs have been classified as an advanced therapy medicinal product by the EMA and have orphan designation in the European Union. ![]()