User login
CASE: Psychotic and sleepless
Mr. F, age 30, is referred to our psychiatric outpatient clinic for follow-up care after hospitalization to treat a psychotic episode. His psychotic symptoms started 2 years ago without an identifiable trigger. Mr. F complains of episodic mood symptoms, such as depression, irritability, and angry outbursts; persistent auditory hallucinations (voices calling him names); and persecutory delusions. While in the hospital he was diagnosed with psychotic disorder not otherwise specified and started on olanzapine titrated to 30 mg/d.
During evaluation, Mr. F is depressed and exhibits motor retardation, slow speech, bland affect, impaired short-term memory, and auditory hallucinations. He describes social anxiety and has ideas of reference and problems interpreting facial expressions. He is guarded and suspicious. Although auditory hallucinations and depression affect Mr. F’s daily activities, he is attempting to find a job.
Mr. F has used alcohol since age 16 to escape social difficulties. He says he last used alcohol 1 year ago, but refuses to provide details about how much alcohol he typically consumed. Sporadic cannabis use also started when Mr. F was in his teens.
Mr. F’s symptoms improve with olanzapine, but he complains of weight gain and sedation, so we switch him to aripiprazole, 10 mg/d. Two weeks later he reports feeling jittery and anxious, so we discontinue aripiprazole and start loxapine, 25 mg/d at night, and propranolol, 60 mg/d, for residual akathisia. Despite limited clinical improvement, Mr. F irrationally says he wants to join the Navy. After a week, his psychotic symptoms improve but anxiety persists, so we start clonazepam, 1 mg/d, and oxcarbazepine, 600 mg/d. After 2 weeks he says he feels calmer, but has gained 20 lbs and is constantly tired. Against our advice, Mr. F decides to discontinue loxapine and propranolol, but continues clonazepam and oxcarbazepine.
At his next visit 4 weeks later, Mr. F is in good spirits. He says he is looking for a job as a dental assistant, and shows no apparent signs of psychosis. Mr. F misses his next appointment but returns 3 months later with evident deterioration in his general appearance. He says he is having difficulty sleeping and is depressed, stating “I just lay in bed; I don’t want to deal with life.” He is withdrawn and unwilling to elaborate on his personal problems but asks for a refill of clonazepam and oxcarbazepine, which we provide.
The authors’ observations
Sleep disturbances, including poor sleep efficiency, increased sleep-onset latency, decreased rapid eye movement (REM) sleep latency, and decreased stage 4 of non-REM sleep, occur in 16% to 30% of patients with schizophrenia and are associated with reduced quality of life and poor coping skills.1 Sleep-onset and sleep maintenance problems and sleep-wake reversal generally persist despite antipsychotic treatment.2,3
Slow-wave sleep deficiency can lead to negative symptoms and memory deficits in patients with schizophrenia because4:
- declarative and procedural memory consolidation are associated with slow-wave and stage 2 sleep, respectively
- procedural learning and visual spatial memory are correlated with delta power in slow-wave sleep.3,8
Acute psychosis exacerbations are associated with restless, agitated sleep. Insomnia often is an early warning sign of clinical relapse.5 The etiology of sleep dysfunction in schizophrenia is unknown, but glutamatergic action through N-methyl-d-aspartate receptors, the GABA system,6 and the serotonin system7 have been implicated.
Relapse to alcohol could trigger an exacerbation of Mr. F’s illness; however, he continues to deny alcohol or drug use and we could not identify any evidence of alcohol use at his last visit.
HISTORY: Strange behavior
Mr. F is a first-generation immigrant from Venezuela. He has a general educational development diploma and an associate’s degree. He says he has worked as a dental assistant but lost his job after a driving under the influence charge a year ago. Subsequently, he could not remain employed for long. He lives with his parents.
When Mr. F returns to the clinic 5 months later, he has lost 20 lbs and complains of anxiety and lack of sleep. With stooped posture, slow movements, and a mood-incongruent smile, he admits he ran out of medications and asks for refills, which we provide. He appears somewhat bizarre, wearing a loosely fitting baseball cap that covers his direct field of vision. Mr. F admits that he has been pulling out his hair. His thought process is impoverished and his answers are guarded and evasive. He rejects our recommendation of an antipsychotic; the only medications he is willing to continue are oxcarbazepine and clonazepam.
The authors’ observations
Treatment strategies for sleep disorders in patients with schizophrenia mainly target behavioral aspects of sleep, such as sleep onset and total sleep time, and rarely correct polysomnographic disturbances. Commonly used medications include atypical antipsychotics, benzodiazepines, zolpidem, zopiclone, and antidepressants with sedative properties (Table 1).1 However, new insights on sleep architecture patterns in these individuals have directed focus on other medications. Although antipsychotics, GABAA modulators, and melatonin provide some sleep benefits, none of these agents fully address characteristic sleep disturbances found in patients with schizophrenia.
Recent research has looked at GABAB modulators because of their unique function. GABAB receptors are located on pre-synaptic dopaminergic terminals and inhibit dopamine release and modulate glutamatergic regulation of dopamine. In the glutamate hypofunction model of psychosis, a GABAB agonist would cause disinhibition of glutamate modulation of mesolimbic dopamine and reversal of GABA transmission in the ventral tegmental area.9 Baclofen and γ-hydroxybutyric acid (GHB) currently are the only FDA-approved GABAB receptor agonists. Overall, trials of baclofen have not shown benefit for sleep disturbances in patients with schizophrenia,10,11 perhaps because of the drug’s poor liposolubility and consequent inability to cross the blood-brain barrier. Although hydrophilic like baclofen, GHB, which is also known as sodium oxybate and is FDA-approved for cataplexy due to narcolepsy, might have an advantage because of carrier-mediated transfer across the blood-brain barrier. GHB is thought to act directly as a neurotransmitter but also interacts with dopamine via the GHB receptor and with the GABAB receptor after it is converted to extracellular GABA.
Table 1
Schizophrenia and sleep dysfunction: The effect of psychotropics
| Medication/class | Comments |
|---|---|
| Atypical antipsychotics | In the CATIE study, a large proportion of patients had sleep problems despite antipsychotic treatment Atypicals may improve sleep acutely, but do not normalize it The long-term effects of atypicals on sleep architecture in schizophrenia are unclear; some studies show improved slow-wave sleep but in others slow-wave sleep is reduced |
| GABAA modulators (benzodiazepines, zolpidem, zopiclone) | Decrease sleep latency and nocturnal awakening Do not increase slow-wave sleep and overall sleep quality Decrease slow-wave sleep and REM sleep in rats May impair sleep architecture and cognition |
| Melatonin and modafinil | Melatonin may be useful for improving subjective sleep in patients with schizophrenia, although it does not improve slow-wave sleep parameters Modafinil may enhance cognition |
| GABAB receptor agonists | Few trials in humans but animal studies support a potential therapeutic role Minimal impact on REM sleep Increase slow-wave sleep Human studies with the GABAB agonist GHB show improvement in sleep architecture and subjective sleep |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness; GHB: γ-hydroxybutyric acid; REM: rapid eye movement | |
| Source:Reference 1 | |
OUTCOME: A trip cut short
Mr. F does not return to the clinic as scheduled, but 2 months later the U.S. consulate of a Western European country contacts us because Mr. F had a bottle of oxcarbazepine with our contact information. After Mr. F returns to the United States, he tells us his story.
After his last outpatient visit, Mr. F relapsed on alcohol, became despondent over his weakness, and searched for a way to escape his alcohol cravings. He came up with a plan to relocate to an Islamic Middle Eastern country where alcohol is banned and its use heavily punished. Mr. F bought a one-way airplane ticket through a Western Europe connection and departed 7 days later without notifying his family or psychiatrist.
Mr. F’s flight to Western Europe was uneventful. After landing for a connecting flight, his mood improved, his outlook became hopeful, and his auditory hallucinations changed from derogatory to supportive. However, Mr. F became despondent after being barred from his next flight because he did not have a return ticket. He was stranded in the airport with little money and no extra clothing, only his passport and laptop. He slept in the airport and after 3 days set off into the city. Mr. F navigated subway stations, ate at soup kitchens, and sought shelter in hotel lobbies and churches. One week after Mr. F left the airport, the police detained him for disorganized behavior and refusing to vacate a church. He was transported to a hospital, admitted to the psychiatric unit for catatonia, and stabilized on olanzapine, 20 mg/d.
After 1 week, Mr. F was returned to the United States and hospitalized for further evaluation and treatment. On his first day back, Mr. F’s disorganized process appeared to improve. He was euthymic and reported good sleep, tolerable anxiety, and infrequent derogatory auditory hallucinations that were low in volume. On day 3, Mr. F’s mood deteriorated moderately. He became depressed and again experienced derogatory auditory hallucinations. He was internally preoccupied and showed reduced affect and psychomotor activity. Mr. F was discharged the next day to a state-run respite program with a structured plan for psychiatric follow-up, social services, and sobriety maintenance. He remained on olanzapine, 20 mg/d, because we anticipated he would need an adjustment period after his uncommon journey.
The authors’ observations
Psychotic symptoms occurring during long-distance trips have been well described in psychiatric literature. Westbound travel could exacerbate depression. Emerging mania has been documented in eastbound flights, which could be related to sleep deprivation.12,13 The incidence of psychotic exacerbations is correlated with the number of time zones crossed.12
A change in environment, unfamiliar surroundings, presence of strangers, physical inactivity, and a sense of isolation all contribute to jet lag syndrome. Long-distance air travel also disrupts zeitgebers, environmental cues that induce adjustments in the internal body clock.12,14 The body clock is controlled mainly by the SCN in the hypothalamus, which is primarily regulated by the light/dark cycle via melatonin secretion (Figure).
Endogenous changes in circadian rhythms and melatonin secretion abnormalities are present in the pathophysiological mechanism of several psychiatric disorders, including depression, bipolar disorder, and schizophrenia. Trbovic hypothesized that in essence schizophrenia could be a sleep disorder and SCN dysfunction may contribute to the pathogenesis of schizophrenia.15 Several research findings support this hypothesis (Table 2). Recent evidence suggests that abnormal circadian melatonin metabolism may be directly related to the schizophrenia pathophysiology.16 Because melatonin production is regulated by the SCN and jet lag resets the melatonin cycle, a defective SCN may not respond well to such adjustments.
Mr. F’s symptomatology is illustrative of the jet lag scenario. His auditory hallucinations became “more supportive” and helpful during his eastbound flight, whereas after his return to the United States, depression was the predominant mood symptom. Psychotic exacerbation also was noticeable after his return.
There are no recommended treatments for psychosis related to jet lag. Antipsychotics often are used, although there is no accepted agent of choice. Treatment of jet lag includes addressing sleep loss and desynchronization.17 Medications suggested for treatment of sleep loss are antihistamines (H1 receptor antagonists), benzodiazepines, and imidazopyridines (zolpidem, zopiclone). Light therapy or administration of melatonin, ramelteon, or agomelatine can help jet-lagged patients resynchronize with the environment.
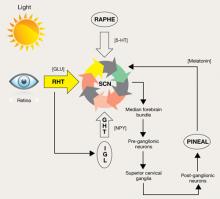
Figure: Pathways for light: Circadian timing system
Photic information reaches the suprachiasmatic nucleus (SCN) through the retinohypothalamic tract (RHT), which uses glutamate (GLU) as a neurotransmitter. A multisynaptic indirect pathway also carries photic information to the SCN. This indirect route arises from the RHT, projects through the intergeniculate leaflet (IGL) of the lateral geniculate nucleus, and finally, the geniculohypothalamic tract (GHT). Neuropeptide Y (NPY) is the neurotransmitter of the GHT. Serotoninergic (5-HT) input to the SCN arrives from the dorsal raphe nuclei. Melatonin, produced in the pineal gland, exerts its effect on circadian timing by feeding back onto the SCN.
Source: Reprinted with permission from reference 14Table 2
Suprachiasmatic nucleus dysfunction may have a role in schizophrenia
| Consequences of SCN dysfunction | Findings relevant to schizophrenia |
|---|---|
| Circadian pattern abnormalities | Individuals with schizophrenia do not have a characteristic circadian pattern of melatonin secretiona Actigraphic studies confirm that patients with schizophrenia have abnormal circadian rhythm activitiesb-d |
| Dopaminergic system abnormalities | The fetal dopaminergic system and D1 dopamine receptors may be involved in the process of synchronizing the SCNe,f |
| Jet lag symptomatology | Jet lag can exacerbate psychiatric disorders,g which suggests that in these patients the SCN is not capable of adjustment |
| Pathologic daytime sleep | Saccadic eye movements in patients with schizophrenia suggest they may be experiencing remnants of REM sleep, supporting the notion that these patients may have dream states during wakefulness |
| REM: rapid eye movement; SCN: suprachiasmatic nucleus | |
| Source: a. Bersani G, Mameli M, Garavini A, et al. Reduction of night/day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Neuro Endocrinol Lett. 2003;24(3-4):181-184. b. Poyurovsky M, Nave R, Epstein R, et al. Actigraphic monitoring (actigraphy) of circadian locomotor activity in schizophrenic patients with acute neuroleptic-induced akathisia. Eur Neuropsychopharmacol. 2000;10(3):171-176. c. Haug HJ, Wirz-Justice A, Rössler W. Actigraphy to measure day structure as a therapeutic variable in the treatment of schizophrenic patients. Acta Psychiatr Scand Suppl. 2000;(407):91-95. d. Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39(3):251-259. e. Strother WN, Norman AB, Lehman MN. D1-dopamine receptor binding and tyrosine hydroxylase-immunoreactivity in the fetal and neonatal hamster suprachiasmatic nucleus. Brain Res Dev Brain Res. 1998;106(1-2):137-144. f. Viswanathan N, Weaver DR, Reppert SM, et al. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14(9):5393-5398. g. Katz G, Durst R, Zislin J, et al. Jet lag causing or exacerbating psychiatric disorders. Harefuah. 2000;138(10):809-812, 912. | |
Related Resources
- Klein DC, Moore R, Reppert SM, eds. Suprachiasmatic nucleus: the mind’s clock. New York, NY: Oxford University Press; 1991.
- Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13.
Drug Brand Names
- Agomelatine • Valdoxan
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Clonazepam • Klonopin
- γ-hydroxybutyric acid, sodium oxybate • Xyrem
- Loxapine • Loxitane
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Propranolol • Inderal
- Ramelteon • Rozerem
- Zolpidem • Ambien
- Zoplicone • Lunesta
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23(8):631-669.
2. Poulin J, Daoust AM, Forest G, et al. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophr Res. 2003;62:147-153.
3. Ferrarelli F, Huber R, Peterson MJ. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483-492.
4. Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939-962.
5. Haffmans P, Hoencamp E, Knegtering HJ, et al. Sleep disturbance in schizophrenia. Br J Psychiatry. 1994;165(5):697-698.
6. Wisor J, Morairty S, Huynh N, et al. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141(1):371-378.
7. Benson KL, Faull KF, Zarcone VP. Evidence for the role of serotonin in the regulation of slow wave sleep in schizophrenia. Sleep. 1991;14(2):133-139.
8. Göder R, Boigs M, Braun S, et al. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res. 2004;38:591-599.
9. Harte M, O’Connor WT. Evidence for a selective prefrontal cortical GABA(B) receptor mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 2005;130(1):215-222.
10. Garbutt JC, van Kammen DP. The interaction between GABA and dopamine: implications for schizophrenia. Schizophr Bull. 1983;9(3):336-353.
11. Finnimore A, Roebuck M, Sajkov D, et al. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J. 1995;8(2):230-234.
12. Jahuar P, Weller MP. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Br J Psychiatry. 1982;140:231-234.
13. Katz G, Durst R, Zislin Y, et al. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 2001;56(1):20-23.
14. Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611-1616.
15. Trbovic SM. Schizophrenia as a possible dysfunction of the suprachiasmatic nucleus. Med Hypotheses. 2010;74:127-131.
16. Bersani G, Mameli M, Garavini A, et al. Reduction of night/ day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Nuero Endocrinol Lett. 2003;24(3-4):181-184.
17. Brown GM, Pandi-Perumal SR, Trakht I, et al. Melatonin and its relevance to jet lag. Travel Med Infect Dis. 2009;7:69-81.
CASE: Psychotic and sleepless
Mr. F, age 30, is referred to our psychiatric outpatient clinic for follow-up care after hospitalization to treat a psychotic episode. His psychotic symptoms started 2 years ago without an identifiable trigger. Mr. F complains of episodic mood symptoms, such as depression, irritability, and angry outbursts; persistent auditory hallucinations (voices calling him names); and persecutory delusions. While in the hospital he was diagnosed with psychotic disorder not otherwise specified and started on olanzapine titrated to 30 mg/d.
During evaluation, Mr. F is depressed and exhibits motor retardation, slow speech, bland affect, impaired short-term memory, and auditory hallucinations. He describes social anxiety and has ideas of reference and problems interpreting facial expressions. He is guarded and suspicious. Although auditory hallucinations and depression affect Mr. F’s daily activities, he is attempting to find a job.
Mr. F has used alcohol since age 16 to escape social difficulties. He says he last used alcohol 1 year ago, but refuses to provide details about how much alcohol he typically consumed. Sporadic cannabis use also started when Mr. F was in his teens.
Mr. F’s symptoms improve with olanzapine, but he complains of weight gain and sedation, so we switch him to aripiprazole, 10 mg/d. Two weeks later he reports feeling jittery and anxious, so we discontinue aripiprazole and start loxapine, 25 mg/d at night, and propranolol, 60 mg/d, for residual akathisia. Despite limited clinical improvement, Mr. F irrationally says he wants to join the Navy. After a week, his psychotic symptoms improve but anxiety persists, so we start clonazepam, 1 mg/d, and oxcarbazepine, 600 mg/d. After 2 weeks he says he feels calmer, but has gained 20 lbs and is constantly tired. Against our advice, Mr. F decides to discontinue loxapine and propranolol, but continues clonazepam and oxcarbazepine.
At his next visit 4 weeks later, Mr. F is in good spirits. He says he is looking for a job as a dental assistant, and shows no apparent signs of psychosis. Mr. F misses his next appointment but returns 3 months later with evident deterioration in his general appearance. He says he is having difficulty sleeping and is depressed, stating “I just lay in bed; I don’t want to deal with life.” He is withdrawn and unwilling to elaborate on his personal problems but asks for a refill of clonazepam and oxcarbazepine, which we provide.
The authors’ observations
Sleep disturbances, including poor sleep efficiency, increased sleep-onset latency, decreased rapid eye movement (REM) sleep latency, and decreased stage 4 of non-REM sleep, occur in 16% to 30% of patients with schizophrenia and are associated with reduced quality of life and poor coping skills.1 Sleep-onset and sleep maintenance problems and sleep-wake reversal generally persist despite antipsychotic treatment.2,3
Slow-wave sleep deficiency can lead to negative symptoms and memory deficits in patients with schizophrenia because4:
- declarative and procedural memory consolidation are associated with slow-wave and stage 2 sleep, respectively
- procedural learning and visual spatial memory are correlated with delta power in slow-wave sleep.3,8
Acute psychosis exacerbations are associated with restless, agitated sleep. Insomnia often is an early warning sign of clinical relapse.5 The etiology of sleep dysfunction in schizophrenia is unknown, but glutamatergic action through N-methyl-d-aspartate receptors, the GABA system,6 and the serotonin system7 have been implicated.
Relapse to alcohol could trigger an exacerbation of Mr. F’s illness; however, he continues to deny alcohol or drug use and we could not identify any evidence of alcohol use at his last visit.
HISTORY: Strange behavior
Mr. F is a first-generation immigrant from Venezuela. He has a general educational development diploma and an associate’s degree. He says he has worked as a dental assistant but lost his job after a driving under the influence charge a year ago. Subsequently, he could not remain employed for long. He lives with his parents.
When Mr. F returns to the clinic 5 months later, he has lost 20 lbs and complains of anxiety and lack of sleep. With stooped posture, slow movements, and a mood-incongruent smile, he admits he ran out of medications and asks for refills, which we provide. He appears somewhat bizarre, wearing a loosely fitting baseball cap that covers his direct field of vision. Mr. F admits that he has been pulling out his hair. His thought process is impoverished and his answers are guarded and evasive. He rejects our recommendation of an antipsychotic; the only medications he is willing to continue are oxcarbazepine and clonazepam.
The authors’ observations
Treatment strategies for sleep disorders in patients with schizophrenia mainly target behavioral aspects of sleep, such as sleep onset and total sleep time, and rarely correct polysomnographic disturbances. Commonly used medications include atypical antipsychotics, benzodiazepines, zolpidem, zopiclone, and antidepressants with sedative properties (Table 1).1 However, new insights on sleep architecture patterns in these individuals have directed focus on other medications. Although antipsychotics, GABAA modulators, and melatonin provide some sleep benefits, none of these agents fully address characteristic sleep disturbances found in patients with schizophrenia.
Recent research has looked at GABAB modulators because of their unique function. GABAB receptors are located on pre-synaptic dopaminergic terminals and inhibit dopamine release and modulate glutamatergic regulation of dopamine. In the glutamate hypofunction model of psychosis, a GABAB agonist would cause disinhibition of glutamate modulation of mesolimbic dopamine and reversal of GABA transmission in the ventral tegmental area.9 Baclofen and γ-hydroxybutyric acid (GHB) currently are the only FDA-approved GABAB receptor agonists. Overall, trials of baclofen have not shown benefit for sleep disturbances in patients with schizophrenia,10,11 perhaps because of the drug’s poor liposolubility and consequent inability to cross the blood-brain barrier. Although hydrophilic like baclofen, GHB, which is also known as sodium oxybate and is FDA-approved for cataplexy due to narcolepsy, might have an advantage because of carrier-mediated transfer across the blood-brain barrier. GHB is thought to act directly as a neurotransmitter but also interacts with dopamine via the GHB receptor and with the GABAB receptor after it is converted to extracellular GABA.
Table 1
Schizophrenia and sleep dysfunction: The effect of psychotropics
| Medication/class | Comments |
|---|---|
| Atypical antipsychotics | In the CATIE study, a large proportion of patients had sleep problems despite antipsychotic treatment Atypicals may improve sleep acutely, but do not normalize it The long-term effects of atypicals on sleep architecture in schizophrenia are unclear; some studies show improved slow-wave sleep but in others slow-wave sleep is reduced |
| GABAA modulators (benzodiazepines, zolpidem, zopiclone) | Decrease sleep latency and nocturnal awakening Do not increase slow-wave sleep and overall sleep quality Decrease slow-wave sleep and REM sleep in rats May impair sleep architecture and cognition |
| Melatonin and modafinil | Melatonin may be useful for improving subjective sleep in patients with schizophrenia, although it does not improve slow-wave sleep parameters Modafinil may enhance cognition |
| GABAB receptor agonists | Few trials in humans but animal studies support a potential therapeutic role Minimal impact on REM sleep Increase slow-wave sleep Human studies with the GABAB agonist GHB show improvement in sleep architecture and subjective sleep |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness; GHB: γ-hydroxybutyric acid; REM: rapid eye movement | |
| Source:Reference 1 | |
OUTCOME: A trip cut short
Mr. F does not return to the clinic as scheduled, but 2 months later the U.S. consulate of a Western European country contacts us because Mr. F had a bottle of oxcarbazepine with our contact information. After Mr. F returns to the United States, he tells us his story.
After his last outpatient visit, Mr. F relapsed on alcohol, became despondent over his weakness, and searched for a way to escape his alcohol cravings. He came up with a plan to relocate to an Islamic Middle Eastern country where alcohol is banned and its use heavily punished. Mr. F bought a one-way airplane ticket through a Western Europe connection and departed 7 days later without notifying his family or psychiatrist.
Mr. F’s flight to Western Europe was uneventful. After landing for a connecting flight, his mood improved, his outlook became hopeful, and his auditory hallucinations changed from derogatory to supportive. However, Mr. F became despondent after being barred from his next flight because he did not have a return ticket. He was stranded in the airport with little money and no extra clothing, only his passport and laptop. He slept in the airport and after 3 days set off into the city. Mr. F navigated subway stations, ate at soup kitchens, and sought shelter in hotel lobbies and churches. One week after Mr. F left the airport, the police detained him for disorganized behavior and refusing to vacate a church. He was transported to a hospital, admitted to the psychiatric unit for catatonia, and stabilized on olanzapine, 20 mg/d.
After 1 week, Mr. F was returned to the United States and hospitalized for further evaluation and treatment. On his first day back, Mr. F’s disorganized process appeared to improve. He was euthymic and reported good sleep, tolerable anxiety, and infrequent derogatory auditory hallucinations that were low in volume. On day 3, Mr. F’s mood deteriorated moderately. He became depressed and again experienced derogatory auditory hallucinations. He was internally preoccupied and showed reduced affect and psychomotor activity. Mr. F was discharged the next day to a state-run respite program with a structured plan for psychiatric follow-up, social services, and sobriety maintenance. He remained on olanzapine, 20 mg/d, because we anticipated he would need an adjustment period after his uncommon journey.
The authors’ observations
Psychotic symptoms occurring during long-distance trips have been well described in psychiatric literature. Westbound travel could exacerbate depression. Emerging mania has been documented in eastbound flights, which could be related to sleep deprivation.12,13 The incidence of psychotic exacerbations is correlated with the number of time zones crossed.12
A change in environment, unfamiliar surroundings, presence of strangers, physical inactivity, and a sense of isolation all contribute to jet lag syndrome. Long-distance air travel also disrupts zeitgebers, environmental cues that induce adjustments in the internal body clock.12,14 The body clock is controlled mainly by the SCN in the hypothalamus, which is primarily regulated by the light/dark cycle via melatonin secretion (Figure).
Endogenous changes in circadian rhythms and melatonin secretion abnormalities are present in the pathophysiological mechanism of several psychiatric disorders, including depression, bipolar disorder, and schizophrenia. Trbovic hypothesized that in essence schizophrenia could be a sleep disorder and SCN dysfunction may contribute to the pathogenesis of schizophrenia.15 Several research findings support this hypothesis (Table 2). Recent evidence suggests that abnormal circadian melatonin metabolism may be directly related to the schizophrenia pathophysiology.16 Because melatonin production is regulated by the SCN and jet lag resets the melatonin cycle, a defective SCN may not respond well to such adjustments.
Mr. F’s symptomatology is illustrative of the jet lag scenario. His auditory hallucinations became “more supportive” and helpful during his eastbound flight, whereas after his return to the United States, depression was the predominant mood symptom. Psychotic exacerbation also was noticeable after his return.
There are no recommended treatments for psychosis related to jet lag. Antipsychotics often are used, although there is no accepted agent of choice. Treatment of jet lag includes addressing sleep loss and desynchronization.17 Medications suggested for treatment of sleep loss are antihistamines (H1 receptor antagonists), benzodiazepines, and imidazopyridines (zolpidem, zopiclone). Light therapy or administration of melatonin, ramelteon, or agomelatine can help jet-lagged patients resynchronize with the environment.
Figure: Pathways for light: Circadian timing system
Photic information reaches the suprachiasmatic nucleus (SCN) through the retinohypothalamic tract (RHT), which uses glutamate (GLU) as a neurotransmitter. A multisynaptic indirect pathway also carries photic information to the SCN. This indirect route arises from the RHT, projects through the intergeniculate leaflet (IGL) of the lateral geniculate nucleus, and finally, the geniculohypothalamic tract (GHT). Neuropeptide Y (NPY) is the neurotransmitter of the GHT. Serotoninergic (5-HT) input to the SCN arrives from the dorsal raphe nuclei. Melatonin, produced in the pineal gland, exerts its effect on circadian timing by feeding back onto the SCN.
Source: Reprinted with permission from reference 14Table 2
Suprachiasmatic nucleus dysfunction may have a role in schizophrenia
| Consequences of SCN dysfunction | Findings relevant to schizophrenia |
|---|---|
| Circadian pattern abnormalities | Individuals with schizophrenia do not have a characteristic circadian pattern of melatonin secretiona Actigraphic studies confirm that patients with schizophrenia have abnormal circadian rhythm activitiesb-d |
| Dopaminergic system abnormalities | The fetal dopaminergic system and D1 dopamine receptors may be involved in the process of synchronizing the SCNe,f |
| Jet lag symptomatology | Jet lag can exacerbate psychiatric disorders,g which suggests that in these patients the SCN is not capable of adjustment |
| Pathologic daytime sleep | Saccadic eye movements in patients with schizophrenia suggest they may be experiencing remnants of REM sleep, supporting the notion that these patients may have dream states during wakefulness |
| REM: rapid eye movement; SCN: suprachiasmatic nucleus | |
| Source: a. Bersani G, Mameli M, Garavini A, et al. Reduction of night/day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Neuro Endocrinol Lett. 2003;24(3-4):181-184. b. Poyurovsky M, Nave R, Epstein R, et al. Actigraphic monitoring (actigraphy) of circadian locomotor activity in schizophrenic patients with acute neuroleptic-induced akathisia. Eur Neuropsychopharmacol. 2000;10(3):171-176. c. Haug HJ, Wirz-Justice A, Rössler W. Actigraphy to measure day structure as a therapeutic variable in the treatment of schizophrenic patients. Acta Psychiatr Scand Suppl. 2000;(407):91-95. d. Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39(3):251-259. e. Strother WN, Norman AB, Lehman MN. D1-dopamine receptor binding and tyrosine hydroxylase-immunoreactivity in the fetal and neonatal hamster suprachiasmatic nucleus. Brain Res Dev Brain Res. 1998;106(1-2):137-144. f. Viswanathan N, Weaver DR, Reppert SM, et al. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14(9):5393-5398. g. Katz G, Durst R, Zislin J, et al. Jet lag causing or exacerbating psychiatric disorders. Harefuah. 2000;138(10):809-812, 912. | |
Related Resources
- Klein DC, Moore R, Reppert SM, eds. Suprachiasmatic nucleus: the mind’s clock. New York, NY: Oxford University Press; 1991.
- Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13.
Drug Brand Names
- Agomelatine • Valdoxan
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Clonazepam • Klonopin
- γ-hydroxybutyric acid, sodium oxybate • Xyrem
- Loxapine • Loxitane
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Propranolol • Inderal
- Ramelteon • Rozerem
- Zolpidem • Ambien
- Zoplicone • Lunesta
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Psychotic and sleepless
Mr. F, age 30, is referred to our psychiatric outpatient clinic for follow-up care after hospitalization to treat a psychotic episode. His psychotic symptoms started 2 years ago without an identifiable trigger. Mr. F complains of episodic mood symptoms, such as depression, irritability, and angry outbursts; persistent auditory hallucinations (voices calling him names); and persecutory delusions. While in the hospital he was diagnosed with psychotic disorder not otherwise specified and started on olanzapine titrated to 30 mg/d.
During evaluation, Mr. F is depressed and exhibits motor retardation, slow speech, bland affect, impaired short-term memory, and auditory hallucinations. He describes social anxiety and has ideas of reference and problems interpreting facial expressions. He is guarded and suspicious. Although auditory hallucinations and depression affect Mr. F’s daily activities, he is attempting to find a job.
Mr. F has used alcohol since age 16 to escape social difficulties. He says he last used alcohol 1 year ago, but refuses to provide details about how much alcohol he typically consumed. Sporadic cannabis use also started when Mr. F was in his teens.
Mr. F’s symptoms improve with olanzapine, but he complains of weight gain and sedation, so we switch him to aripiprazole, 10 mg/d. Two weeks later he reports feeling jittery and anxious, so we discontinue aripiprazole and start loxapine, 25 mg/d at night, and propranolol, 60 mg/d, for residual akathisia. Despite limited clinical improvement, Mr. F irrationally says he wants to join the Navy. After a week, his psychotic symptoms improve but anxiety persists, so we start clonazepam, 1 mg/d, and oxcarbazepine, 600 mg/d. After 2 weeks he says he feels calmer, but has gained 20 lbs and is constantly tired. Against our advice, Mr. F decides to discontinue loxapine and propranolol, but continues clonazepam and oxcarbazepine.
At his next visit 4 weeks later, Mr. F is in good spirits. He says he is looking for a job as a dental assistant, and shows no apparent signs of psychosis. Mr. F misses his next appointment but returns 3 months later with evident deterioration in his general appearance. He says he is having difficulty sleeping and is depressed, stating “I just lay in bed; I don’t want to deal with life.” He is withdrawn and unwilling to elaborate on his personal problems but asks for a refill of clonazepam and oxcarbazepine, which we provide.
The authors’ observations
Sleep disturbances, including poor sleep efficiency, increased sleep-onset latency, decreased rapid eye movement (REM) sleep latency, and decreased stage 4 of non-REM sleep, occur in 16% to 30% of patients with schizophrenia and are associated with reduced quality of life and poor coping skills.1 Sleep-onset and sleep maintenance problems and sleep-wake reversal generally persist despite antipsychotic treatment.2,3
Slow-wave sleep deficiency can lead to negative symptoms and memory deficits in patients with schizophrenia because4:
- declarative and procedural memory consolidation are associated with slow-wave and stage 2 sleep, respectively
- procedural learning and visual spatial memory are correlated with delta power in slow-wave sleep.3,8
Acute psychosis exacerbations are associated with restless, agitated sleep. Insomnia often is an early warning sign of clinical relapse.5 The etiology of sleep dysfunction in schizophrenia is unknown, but glutamatergic action through N-methyl-d-aspartate receptors, the GABA system,6 and the serotonin system7 have been implicated.
Relapse to alcohol could trigger an exacerbation of Mr. F’s illness; however, he continues to deny alcohol or drug use and we could not identify any evidence of alcohol use at his last visit.
HISTORY: Strange behavior
Mr. F is a first-generation immigrant from Venezuela. He has a general educational development diploma and an associate’s degree. He says he has worked as a dental assistant but lost his job after a driving under the influence charge a year ago. Subsequently, he could not remain employed for long. He lives with his parents.
When Mr. F returns to the clinic 5 months later, he has lost 20 lbs and complains of anxiety and lack of sleep. With stooped posture, slow movements, and a mood-incongruent smile, he admits he ran out of medications and asks for refills, which we provide. He appears somewhat bizarre, wearing a loosely fitting baseball cap that covers his direct field of vision. Mr. F admits that he has been pulling out his hair. His thought process is impoverished and his answers are guarded and evasive. He rejects our recommendation of an antipsychotic; the only medications he is willing to continue are oxcarbazepine and clonazepam.
The authors’ observations
Treatment strategies for sleep disorders in patients with schizophrenia mainly target behavioral aspects of sleep, such as sleep onset and total sleep time, and rarely correct polysomnographic disturbances. Commonly used medications include atypical antipsychotics, benzodiazepines, zolpidem, zopiclone, and antidepressants with sedative properties (Table 1).1 However, new insights on sleep architecture patterns in these individuals have directed focus on other medications. Although antipsychotics, GABAA modulators, and melatonin provide some sleep benefits, none of these agents fully address characteristic sleep disturbances found in patients with schizophrenia.
Recent research has looked at GABAB modulators because of their unique function. GABAB receptors are located on pre-synaptic dopaminergic terminals and inhibit dopamine release and modulate glutamatergic regulation of dopamine. In the glutamate hypofunction model of psychosis, a GABAB agonist would cause disinhibition of glutamate modulation of mesolimbic dopamine and reversal of GABA transmission in the ventral tegmental area.9 Baclofen and γ-hydroxybutyric acid (GHB) currently are the only FDA-approved GABAB receptor agonists. Overall, trials of baclofen have not shown benefit for sleep disturbances in patients with schizophrenia,10,11 perhaps because of the drug’s poor liposolubility and consequent inability to cross the blood-brain barrier. Although hydrophilic like baclofen, GHB, which is also known as sodium oxybate and is FDA-approved for cataplexy due to narcolepsy, might have an advantage because of carrier-mediated transfer across the blood-brain barrier. GHB is thought to act directly as a neurotransmitter but also interacts with dopamine via the GHB receptor and with the GABAB receptor after it is converted to extracellular GABA.
Table 1
Schizophrenia and sleep dysfunction: The effect of psychotropics
| Medication/class | Comments |
|---|---|
| Atypical antipsychotics | In the CATIE study, a large proportion of patients had sleep problems despite antipsychotic treatment Atypicals may improve sleep acutely, but do not normalize it The long-term effects of atypicals on sleep architecture in schizophrenia are unclear; some studies show improved slow-wave sleep but in others slow-wave sleep is reduced |
| GABAA modulators (benzodiazepines, zolpidem, zopiclone) | Decrease sleep latency and nocturnal awakening Do not increase slow-wave sleep and overall sleep quality Decrease slow-wave sleep and REM sleep in rats May impair sleep architecture and cognition |
| Melatonin and modafinil | Melatonin may be useful for improving subjective sleep in patients with schizophrenia, although it does not improve slow-wave sleep parameters Modafinil may enhance cognition |
| GABAB receptor agonists | Few trials in humans but animal studies support a potential therapeutic role Minimal impact on REM sleep Increase slow-wave sleep Human studies with the GABAB agonist GHB show improvement in sleep architecture and subjective sleep |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness; GHB: γ-hydroxybutyric acid; REM: rapid eye movement | |
| Source:Reference 1 | |
OUTCOME: A trip cut short
Mr. F does not return to the clinic as scheduled, but 2 months later the U.S. consulate of a Western European country contacts us because Mr. F had a bottle of oxcarbazepine with our contact information. After Mr. F returns to the United States, he tells us his story.
After his last outpatient visit, Mr. F relapsed on alcohol, became despondent over his weakness, and searched for a way to escape his alcohol cravings. He came up with a plan to relocate to an Islamic Middle Eastern country where alcohol is banned and its use heavily punished. Mr. F bought a one-way airplane ticket through a Western Europe connection and departed 7 days later without notifying his family or psychiatrist.
Mr. F’s flight to Western Europe was uneventful. After landing for a connecting flight, his mood improved, his outlook became hopeful, and his auditory hallucinations changed from derogatory to supportive. However, Mr. F became despondent after being barred from his next flight because he did not have a return ticket. He was stranded in the airport with little money and no extra clothing, only his passport and laptop. He slept in the airport and after 3 days set off into the city. Mr. F navigated subway stations, ate at soup kitchens, and sought shelter in hotel lobbies and churches. One week after Mr. F left the airport, the police detained him for disorganized behavior and refusing to vacate a church. He was transported to a hospital, admitted to the psychiatric unit for catatonia, and stabilized on olanzapine, 20 mg/d.
After 1 week, Mr. F was returned to the United States and hospitalized for further evaluation and treatment. On his first day back, Mr. F’s disorganized process appeared to improve. He was euthymic and reported good sleep, tolerable anxiety, and infrequent derogatory auditory hallucinations that were low in volume. On day 3, Mr. F’s mood deteriorated moderately. He became depressed and again experienced derogatory auditory hallucinations. He was internally preoccupied and showed reduced affect and psychomotor activity. Mr. F was discharged the next day to a state-run respite program with a structured plan for psychiatric follow-up, social services, and sobriety maintenance. He remained on olanzapine, 20 mg/d, because we anticipated he would need an adjustment period after his uncommon journey.
The authors’ observations
Psychotic symptoms occurring during long-distance trips have been well described in psychiatric literature. Westbound travel could exacerbate depression. Emerging mania has been documented in eastbound flights, which could be related to sleep deprivation.12,13 The incidence of psychotic exacerbations is correlated with the number of time zones crossed.12
A change in environment, unfamiliar surroundings, presence of strangers, physical inactivity, and a sense of isolation all contribute to jet lag syndrome. Long-distance air travel also disrupts zeitgebers, environmental cues that induce adjustments in the internal body clock.12,14 The body clock is controlled mainly by the SCN in the hypothalamus, which is primarily regulated by the light/dark cycle via melatonin secretion (Figure).
Endogenous changes in circadian rhythms and melatonin secretion abnormalities are present in the pathophysiological mechanism of several psychiatric disorders, including depression, bipolar disorder, and schizophrenia. Trbovic hypothesized that in essence schizophrenia could be a sleep disorder and SCN dysfunction may contribute to the pathogenesis of schizophrenia.15 Several research findings support this hypothesis (Table 2). Recent evidence suggests that abnormal circadian melatonin metabolism may be directly related to the schizophrenia pathophysiology.16 Because melatonin production is regulated by the SCN and jet lag resets the melatonin cycle, a defective SCN may not respond well to such adjustments.
Mr. F’s symptomatology is illustrative of the jet lag scenario. His auditory hallucinations became “more supportive” and helpful during his eastbound flight, whereas after his return to the United States, depression was the predominant mood symptom. Psychotic exacerbation also was noticeable after his return.
There are no recommended treatments for psychosis related to jet lag. Antipsychotics often are used, although there is no accepted agent of choice. Treatment of jet lag includes addressing sleep loss and desynchronization.17 Medications suggested for treatment of sleep loss are antihistamines (H1 receptor antagonists), benzodiazepines, and imidazopyridines (zolpidem, zopiclone). Light therapy or administration of melatonin, ramelteon, or agomelatine can help jet-lagged patients resynchronize with the environment.
Figure: Pathways for light: Circadian timing system
Photic information reaches the suprachiasmatic nucleus (SCN) through the retinohypothalamic tract (RHT), which uses glutamate (GLU) as a neurotransmitter. A multisynaptic indirect pathway also carries photic information to the SCN. This indirect route arises from the RHT, projects through the intergeniculate leaflet (IGL) of the lateral geniculate nucleus, and finally, the geniculohypothalamic tract (GHT). Neuropeptide Y (NPY) is the neurotransmitter of the GHT. Serotoninergic (5-HT) input to the SCN arrives from the dorsal raphe nuclei. Melatonin, produced in the pineal gland, exerts its effect on circadian timing by feeding back onto the SCN.
Source: Reprinted with permission from reference 14Table 2
Suprachiasmatic nucleus dysfunction may have a role in schizophrenia
| Consequences of SCN dysfunction | Findings relevant to schizophrenia |
|---|---|
| Circadian pattern abnormalities | Individuals with schizophrenia do not have a characteristic circadian pattern of melatonin secretiona Actigraphic studies confirm that patients with schizophrenia have abnormal circadian rhythm activitiesb-d |
| Dopaminergic system abnormalities | The fetal dopaminergic system and D1 dopamine receptors may be involved in the process of synchronizing the SCNe,f |
| Jet lag symptomatology | Jet lag can exacerbate psychiatric disorders,g which suggests that in these patients the SCN is not capable of adjustment |
| Pathologic daytime sleep | Saccadic eye movements in patients with schizophrenia suggest they may be experiencing remnants of REM sleep, supporting the notion that these patients may have dream states during wakefulness |
| REM: rapid eye movement; SCN: suprachiasmatic nucleus | |
| Source: a. Bersani G, Mameli M, Garavini A, et al. Reduction of night/day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Neuro Endocrinol Lett. 2003;24(3-4):181-184. b. Poyurovsky M, Nave R, Epstein R, et al. Actigraphic monitoring (actigraphy) of circadian locomotor activity in schizophrenic patients with acute neuroleptic-induced akathisia. Eur Neuropsychopharmacol. 2000;10(3):171-176. c. Haug HJ, Wirz-Justice A, Rössler W. Actigraphy to measure day structure as a therapeutic variable in the treatment of schizophrenic patients. Acta Psychiatr Scand Suppl. 2000;(407):91-95. d. Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39(3):251-259. e. Strother WN, Norman AB, Lehman MN. D1-dopamine receptor binding and tyrosine hydroxylase-immunoreactivity in the fetal and neonatal hamster suprachiasmatic nucleus. Brain Res Dev Brain Res. 1998;106(1-2):137-144. f. Viswanathan N, Weaver DR, Reppert SM, et al. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14(9):5393-5398. g. Katz G, Durst R, Zislin J, et al. Jet lag causing or exacerbating psychiatric disorders. Harefuah. 2000;138(10):809-812, 912. | |
Related Resources
- Klein DC, Moore R, Reppert SM, eds. Suprachiasmatic nucleus: the mind’s clock. New York, NY: Oxford University Press; 1991.
- Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13.
Drug Brand Names
- Agomelatine • Valdoxan
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Clonazepam • Klonopin
- γ-hydroxybutyric acid, sodium oxybate • Xyrem
- Loxapine • Loxitane
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Propranolol • Inderal
- Ramelteon • Rozerem
- Zolpidem • Ambien
- Zoplicone • Lunesta
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23(8):631-669.
2. Poulin J, Daoust AM, Forest G, et al. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophr Res. 2003;62:147-153.
3. Ferrarelli F, Huber R, Peterson MJ. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483-492.
4. Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939-962.
5. Haffmans P, Hoencamp E, Knegtering HJ, et al. Sleep disturbance in schizophrenia. Br J Psychiatry. 1994;165(5):697-698.
6. Wisor J, Morairty S, Huynh N, et al. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141(1):371-378.
7. Benson KL, Faull KF, Zarcone VP. Evidence for the role of serotonin in the regulation of slow wave sleep in schizophrenia. Sleep. 1991;14(2):133-139.
8. Göder R, Boigs M, Braun S, et al. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res. 2004;38:591-599.
9. Harte M, O’Connor WT. Evidence for a selective prefrontal cortical GABA(B) receptor mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 2005;130(1):215-222.
10. Garbutt JC, van Kammen DP. The interaction between GABA and dopamine: implications for schizophrenia. Schizophr Bull. 1983;9(3):336-353.
11. Finnimore A, Roebuck M, Sajkov D, et al. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J. 1995;8(2):230-234.
12. Jahuar P, Weller MP. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Br J Psychiatry. 1982;140:231-234.
13. Katz G, Durst R, Zislin Y, et al. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 2001;56(1):20-23.
14. Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611-1616.
15. Trbovic SM. Schizophrenia as a possible dysfunction of the suprachiasmatic nucleus. Med Hypotheses. 2010;74:127-131.
16. Bersani G, Mameli M, Garavini A, et al. Reduction of night/ day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Nuero Endocrinol Lett. 2003;24(3-4):181-184.
17. Brown GM, Pandi-Perumal SR, Trakht I, et al. Melatonin and its relevance to jet lag. Travel Med Infect Dis. 2009;7:69-81.
1. Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23(8):631-669.
2. Poulin J, Daoust AM, Forest G, et al. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophr Res. 2003;62:147-153.
3. Ferrarelli F, Huber R, Peterson MJ. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483-492.
4. Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939-962.
5. Haffmans P, Hoencamp E, Knegtering HJ, et al. Sleep disturbance in schizophrenia. Br J Psychiatry. 1994;165(5):697-698.
6. Wisor J, Morairty S, Huynh N, et al. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141(1):371-378.
7. Benson KL, Faull KF, Zarcone VP. Evidence for the role of serotonin in the regulation of slow wave sleep in schizophrenia. Sleep. 1991;14(2):133-139.
8. Göder R, Boigs M, Braun S, et al. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res. 2004;38:591-599.
9. Harte M, O’Connor WT. Evidence for a selective prefrontal cortical GABA(B) receptor mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 2005;130(1):215-222.
10. Garbutt JC, van Kammen DP. The interaction between GABA and dopamine: implications for schizophrenia. Schizophr Bull. 1983;9(3):336-353.
11. Finnimore A, Roebuck M, Sajkov D, et al. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J. 1995;8(2):230-234.
12. Jahuar P, Weller MP. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Br J Psychiatry. 1982;140:231-234.
13. Katz G, Durst R, Zislin Y, et al. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 2001;56(1):20-23.
14. Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611-1616.
15. Trbovic SM. Schizophrenia as a possible dysfunction of the suprachiasmatic nucleus. Med Hypotheses. 2010;74:127-131.
16. Bersani G, Mameli M, Garavini A, et al. Reduction of night/ day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Nuero Endocrinol Lett. 2003;24(3-4):181-184.
17. Brown GM, Pandi-Perumal SR, Trakht I, et al. Melatonin and its relevance to jet lag. Travel Med Infect Dis. 2009;7:69-81.