User login
From the Center for Nursing Science & Clinical Inquiry (Dr. McCarthy), and Nutrition Care Division, (Ms. Phipps), Madigan Army Medical Center, Tacoma, WA.
Abstract
- Background: Many controversies exist in the field of nutrition support today, particularly in the critical care environment where nutrition plays a more primary rather than adjunctive role.
- Objective: To provide a brief review of current controversies regarding nutrition therapy in the ICU focusing on the choices regarding the nutrition regimen and the safe, consistent delivery of nutrition as measured by clinical outcomes.
- Methods: Selected areas of controversy are discussed detailing the strengths and weaknesses of the research behind opposing opinions.
- Results: ICU nutrition support controversies include enteral vs parenteral nutrition, use of supplmental parenteral nutrition, protein quantity and quality, and polymeric vs immune-modulating nutrients. Issues surrounding the safety of nutrition support therapy include gastric vs small bowel feeding and trophic vs full feeding. Evidence-based recommendations published by professional societies are presented.
- Conclusion: Understanding a patient’s risk for disease and predicting their response to treatment will assist clinicians with selecting those nutrition interventions that will achieve the best possible outcomes.
According to the National Library of Medicine’s translation of the Hippocratic oath, nowhere does it explicitly say “First, do no harm.” What is written is this: “I will use those dietary regimens which will benefit my patients according to my greatest ability and judgement, and I will do no harm or injustice to them” [1]. In another renowned text, one can find this observation regarding diet by a noted scholar, clinician, and the founder of modern nursing, Florence Nightingale: “Every careful observer of the sick will agree in this that thousands of patients are annually starved in the midst of plenty, from want of attention to the ways which alone make it possible for them to take food” [2,]. While Nightingale was alluding to malnutrition of hospitalized patients, it seems that her real concern may have been the iatrogenic malnutrition that inevitably accompanies hospitalization, even today [3].
From these philosophic texts, we have two ongoing controversies in modern day nutrition therapy identified: (1) what evidence do we have to support the choice of dietary regimens (ie, enteral vs parenteral therapy, timing of supplemental parenteral nutrition, standard vs high protein formula, polymeric vs immune-modulating nutrients) that best serve critically ill patients, and (2) how do we ensure that ICU patients are fed in a safe, consistent, and effective manner (gastric vs small bowel tube placement, gastric residual monitoring or not, trophic vs full feeding) as measured by clinically relevant outcomes? Many controversies exist in the field of nutrition support today [4–7] and a comprehensive discussion of all of them is beyond the scope of this paper. In this paper we will provide a brief review of current controversies focusing on those mentioned above which have only recently been challenged by new rigorous randomized clinical trials (RCTs), and in some cases, subsequent systematic reviews and meta-analyses [8–11].
The Path to Modern Day Nutrition Support Therapy
The field of nutrition support, in general, has expanded greatly over the last 4 decades, but perhaps the most notable advancements have occurred in the critical care environment where efforts have been directed at advancing our understanding of the molecular and biological effects of nutrients in maintaining homeostasis in the critically ill [6]. In recent years, specialized nutrition, delivered by the enteral or parenteral route, was finally recognized for its contribution to important clinical outcomes in the critically ill population [12]. Critical care clinicians have been educated about the advances in nutrition therapy designed to address the unique needs of a vulnerable population where survival is threatened by poor nutritional status upon admission, compromised immune function, weakened respiratory muscles with decreased ventilation capacity, and gastrointestinal (GI) dysfunction [6]. The rapid deterioration seen in these patients is exaggerated by the all too common ICU conditions of systemic inflammatory response syndrome (SIRS), sepsis, hemodynamic instability, respiratory failure, coagulation disorders, and acute kidney injury [13,14].
Beginning in the early 1990s, formulations of enteral nutrition (EN) contained active nutrients that reportedly reduced oxidative damage to cells and tissues, modulated inflammation, and improved feeding tolerance. These benefits are now referred to as the non-nutritive benefits of enteral feeding [15]. For the next 20 years, scientific publications released new results from studies examining the role of omega-3 fatty acids, antioxidant vitamins, minerals such as selenium and zinc, ribonucleotides, and conditionally essential amino acids like glutamine and arginine, in healing and recovery from critical illness. The excitement was summarized succinctly by Hegazi and Wischmeyer in 2011 when they remarked that the modern ICU clinician now has scientific data to guide specialized nutrition therapy, for example, choosing formulas supplemented with anti-inflammatory, immune-modulating, or tolerance-promoting nutrients that have the potential to enhance natural recovery processes and prevent complications [16].
The improvements in nutritional formulas were accompanied by numerous technological advances including bedside devices (electromagnetic enteral access system, real-time image-guided disposable feeding tube, smart feeding pumps with water flush technology) that quickly and safely establish access for small bowel feedings, which help minimize risk of gastric aspiration and ventilator-associated pneumonia, promote tolerance, decrease radiologic exposure, and may reduce nursing time consumed by tube placements, GI dysfunction, and patient discomfort [17–20]. Nasogastric feeding remains the most common first approach, with local practices, contraindications, and ease of placement usually determining the location of the feeding tube [5]. The advancements helped to overcome the many barriers to initiating and maintaining feedings and thus, efforts to feed critically ill patients early and effectively became more routine, along with nurse, patient, and family satisfaction. In conjunction with the innovative approaches to establishing nutrition therapy, practice guidelines published by United States, European, and Canadian nutrition societies became widely available in the past decade with graded evidence-based recommendations for who, when, what, and how to feed, and unique considerations for various critically ill populations [12,21,22]. The tireless efforts by the nutrition societies to provide much needed guidelines for clinicians were appreciated, yet there was a wide range in the grade of the recommendations, with many based on expert opinion alone. In some cases, the research conducted lacked rigor or had missing data with obvious limits to the generalizability of results. Nevertheless, for the 7 years between the publication of the old and newly revised Society of Critical Care Medicine (SCCM)/ American Society of Parenteral and Enteral Nutrition (ASPEN) Guidelines (2016), [12,23] nutrition therapy was a high-priority intervention in most ICUs. The goal was to initiate feeding within 48 hours, select an immune-modulating or other metabolic support formula, and aggressively advance the rate to 80% to 100% of goal to avoid caloric deficit, impaired intestinal integrity, nitrogen losses, and functional impairments [9,24,25]. Nutrition support evolved from adjunctive care to its rightful place in the ABCD mnemonic of early priorities of ICU care: Airway, Breathing, Circulation, Diet.
The 2016 joint publication of the SCCM/ASPEN guidelines includes primarily randomized controlled trial (RCT) data, along with some observational trial data, indexed in any major publication database through December 2013. In these guidelines there were 98 recommendations, of which only 5 were a Level 1A; most of the recommendations were categorized as “expert consensus” [12]. The results of several important clinical trials in the United States and Europe that were underway at the time have since been published and compared to the SCCM/ASPEN relevant recommendations [7]. The results have forced nutrition support clinicians to take a step back and re-examine their practice. For many seasoned clinicians who comprised the nutrition support teams of the 1980s and 1990s, it feels like a return to the basics. Until biology-driven personalized medicine is commonplace and genotype data is readily available to guide nutrition therapy for each critically ill patient, standard enteral feeding that begins slow and proceeds carefully over 5 to 7 days towards 80% of goal caloric intake under judicious monitoring of biochemical and metabolic indices may be the “best practice” today, without risk of harm [15,26]. As in all aspects of clinical care, this practice is not without controversy.
ICU Nutrition Support Controversies Today
Enteral vs Parenteral Nutrition
There is universal consensus that EN is the preferred route for nutrition therapy due to the superior physiological response and both nutritional and non-nutritional benefits [24]. Changes in gut permeability tend to occur as illness progresses and consequences include increased bacterial challenge, risk for multiple organ dysfunction syndrome, and systemic infection. It is best to intervene with nutrition early, defined as within the first 48 hours of ICU admission, while the likelihood of success and opportunity to impact the disease process is greater [12]. Early initiation of feeding provides the necessary nutrients to support gut-associated lymphoid tissue (GALT), mucosal-associated lymphoid tissue (MALT), and preserve gut integrity and microbial diversity [27]. The intestine is an effective barrier against bacteria and intraluminal toxins due to the high rate of enterocyte turnover, the mucus secreted by the goblet cells, and the large amount of protective immunological tissue; 80% of the immunoglobulins are synthesized in the GI tract [28]. Fasting states for procedures or delays in feeding longer than 3 days for any reason may contribute to disruption of intestinal integrity through atrophy and derangements in the physical structure and function of the microvilli and crypts [29]. Intestinal dysfunction leads to increased intestinal permeability and the possibility of bacterial translocation. Intestinal ischemia resulting from shock and sepsis may produce hypoxia and reperfusion injuries further affecting intestinal wall permeability [29]. In surgical patients, early enteral feeding has been found to reduce inflammation, oxidative stress, and the catabolic response to anesthesia and surgical-induced stress, help restore intestinal motility, reverse enteric mucosal atrophy, and improve wound healing [26].
We did not have sufficient data to refute the benefits of EN over PN until the paper by Harvey et al (2014), which reported no difference in mortality or infectious complications in ICU patients receiving EN or PN within 36 hours of admission and for up to 5 days [30]. This was the largest published pragmatic RCT, referred to as the CALORIES trial, which analyzed 2388 patients from 33 ICUs and resulted in controversy over what was an unchallenged approach up until this time. It was only a matter of time before other investigators would set out to confirm or negate this finding, which is what Elke and coworkers (2016) did a few years later [31]. They performed an updated systematic review and meta-analysis to evaluate the overall effect of the route of nutrition (EN versus PN) on clinical outcomes in adult critically ill patients. Similar to the Harvey et al report, they found no difference in mortality between the two routes of nutrition. However, unlike the earlier report, patients receiving EN compared to PN had a significant reduction in the number of infectious complications and ICU length of stay. No significant effect was found for hospital length of stay or days requiring mechanical ventilation. The authors suggest that EN delivery of macronutrients below predefined targets may be responsible as PN is more likely to meet or exceed these targets and overwhelm metabolic capacity in the early days of critical illness [31].
The most recent trial prompting even more discussion about early PN versus early EN in mechanically ventilated ICU patients in shock is the Reignier et al (2018) NUTRIREA-2 trial involving 2410 patients from 44 ICUs in France [32]. The investigators hypothesized that outcomes would be better with early exclusive EN compared to early exclusive PN; their hypothesis was not supported by the results, which found no difference in 28-day mortality or ICU-acquired infections. Also unexpected was the higher cumulative incidence of gastrointestinal complications including vomiting, diarrhea, bowel ischemia, and acute colonic obstruction in the EN group. The trial was stopped early after an interim analysis determined that additional enrollment was not likely to significantly change the results of the trial. Given the similarities between the CALORIES trial and this NUTRIREA-2 trial, clinicians now have mounting evidence that equivalent options for nutrition therapy exist and an appropriate selection should be made based on patient-specific indications and treatment goals. In summary, EN remains preferable to PN for the majority of adult critically ill patients due to crucial support of gut integrity, but the optimal dose or rate of delivery to favorably influence clinical outcomes in the first few days following admission remains unknown.
Use of Supplemental Parenteral Nutrition
Both the nutrition support and bench science communities have learned a great deal about PN over the 4 decades it has been in existence, with the most compelling data coming from more recent trials [31–38]. This is because it has taken many years to recover from the days of hyperalimentation or overfeeding ICU patients by providing excessive calories to meet the elevated energy demands and to reverse the hypercatabolism of critical illness. This approach contributed to the complications of hyperglycemia, hyperlipidemia, increased infectious complications, and liver steatosis, all of which gave PN a negative reputation [37]. We now have adjusted the caloric distribution and the actual formulation of PN using the recent FDA-approved lipid emulsion (Soy, Medium-chain triglycerides, Olive oil, and Fish oil; SMOF) and created protocols for administering it based on specific indications, such as loss of GI integrity or demonstrated intolerance. In general, the advances in PN have led to a safer more therapeutic formulation that has its place in critical illness. Manzanares et al [40] reported a trend toward a decrease in ventilation requirement and mortality when a fish oil–containing lipid emulsion was administered to patients who were receiving nutrition support either enterally or parenterally. The meta-analysis combined all soybean oil–sparing lipid emulsions for comparison with soybean oil and was able to show the trend for improved clinical outcomes with soybean oil–sparing lipid emulsions. The main findings of this meta-analysis were that fish oil–containing lipid emulsions may reduce infections and may be associated with a tendency toward fewer mechanical ventilation days, although not mortality, when compared with soybean oil-based strategies or administration of other alternative lipid emulsions in ICU patients [40]. Recent trial results do not change the recommendation for selecting EN first but do suggest lowering the threshold for using PN when EN alone is insufficient to meet nutrition goals. A systematic review reported no benefit of early supplemental PN over late supplemental PN and cautioned that our continued inability to explain greater infectious morbidity and unresolved organ failure limits any justification for early PN [35].
Protein Quantity and Quality
The practice of providing protein in the range of 1.2–2.0 g/kg actual body weight early in critical illness is suggested by expert consensus in the 2016 SCCM/ASPEN guidelines [12]; however, the evidence for efficacy remains controversial. It is argued that the evidence for benefit comes from observational studies, not from prospective RCTs, and that the patients at high risk are often excluded from study protocols. It has also been suggested that in patients with limited vital organ function increased protein delivery may lead to organ compromise. In a recent (2017) paper, Rooyackers et al discussed the post-hoc analyses of data from the EPANIC Trial stating the statistical correlation between protein intake and outcomes indicate that protein was associated with unfavorable outcomes, possibly by inhibiting autophagy [41].
The nutrition support community may have widely varying approaches to feeding critically ill patients but most experts agree that protein may be the most important macronutrient delivered during critical illness. There is consensus that the hypercatabolism associated with stress induces proteolysis and the loss of lean muscle mass, which may affect clinical and functional outcomes beyond the ICU stay. Using multiple assessment modalities, Puthucheary et al (2013) demonstrated a reduction in the rectus femoris muscle of 12.5% over the first week of hospitalization in the ICU and up to 17.7% by day 10. These numbers imply that sufficient protein of at least 1.2 g/kg/day should be provided to minimize these losses, even if the effect on overall outcome remains unknown [42]. Evidence is lacking for whether or not we can prevent the muscle wasting that occurs in critical illness with increased protein dosing. We also need to better identify the possible risks involved with a high-protein intake at the level of the individual patient. A secondary analysis done by Heyland et al (2013) determined that no specific dose or type of macronutrient was found to be associated with improved outcome [43]. It is clear that more large-scale RCTs of protein/amino acid interventions are needed to prove that these nutrition interventions have favorable effects on clinically important outcomes, including long-term physical function.
Polymeric vs Immune-Modulating Nutrients
The Marik and Zaloga (2008) systematic review on immunonutrition in the critically ill convinced most clinicians that while fish-oil based immunonutrition improves the outcome of medical ICU patients, diets supplemented with arginine, with or without glutamine or fish oils, do not demonstrate an advantage over standard enteral products in general ICU, trauma, or burn patients[44]. What followed these trials examining early formulations of immunonutrition was decades of well-intentioned research dedicated to elucidating the mechanism of action for individual immune-modulating nutrients for various populations, including those with acute lung injury/acute respiratory distress syndrome (ARDS) [45–47], sepsis/systemic inflammatory response syndrome [48–50], head and neck cancer [51], upper and lower GI cancer [52–55], and severe acute pancreatitis [56]. Our understanding of immunonutrition and the administration of this formulation in specific disease conditions has grown considerably yet clinicians are still asking exactly what is the role of immunonutrition and who stands to benefit the most from immune-modulating nutrition therapy. The enteral formulations currently available have a proprietary composition and dosage of individual nutrients which yield unpredictable physiologic effects. In addition, the pervasive problem of underfeeding during hospitalization prevents adequate delivery of physiologic doses of nutrients thus contributing to the widely variable research results.
Prevailing expert opinion today is that the majority of critically ill patients will benefit from nutrition support initiated early and delivered consistently; the standard polymeric formula will suffice for the majority of patients with surgical ICU patients potentially deriving benefit from immunonutrition that supports a reduction in infectious complications [57]. In the recent multiple-treatment meta-analysis performed by Mazaki et al (2015) involving 74 studies and 7572 patients, immunonutrition was ranked first for reducing the incidence of 7 complications according to the surface under the cumulative ranking curve; these were as follows: any infection, 0.86; overall complication, 0.88; mortality, 0.81; wound infection, 0.79; intra-abdominal abscess, 0.98; anastomotic leak, 0.79; and sepsis, 0.92. immunonutrition was ranked second for reducing ventilator-associated pneumonia and catheter-associated urinary tract infection (CAUTI), behind immune-modulating PN. The authors stated that immunonutrition was efficacious for reducing the incidence of complications in GI surgery unrelated to the timing of administration [57]. The 2014 publication of results from the MetaPlus Trial [58]challenged the published recommendations for the use of immunonutrition in the medical critically ill population. This trial used high-protein immunonutrition or standard formula for 301 adult critically ill patients in 14 European ICUs with diagnoses such as pneumonia or infections of the urinary tract, bloodstream, central nervous system, eye, ear, nose or throat, and the skin and soft tissue. Even with higher than average target energy intakes of 70% for the high protein immunonutrition group and 80% for the high protein standard group, there were no statistically significant differences in the primary outcome of new infections, or the secondary outcomes of days on mechanical ventilation, Sequential Organ Failure Assessment scores, or ICU and hospital length of stay. However, the 6-month mortality rate of 28% was higher in the medical subgroup [58]. Using these results, as well as existing publications of negative outcomes in medical ICU patients [44,46], the SCCM/ASPEN Guidelines Committee updated its position in 2016 to suggest that immunonutrition formulations or disease-specific formulations should no longer be used routinely in medical ICU patients, including those with acute lung injury/ARDS [12]. The Committee did suggest that these formulations should be reserved for patients with traumatic brain injury and for surgical ICU patients. The benefit for ICU postoperative patients has been linked to the synergistic effect of fish oil and arginine, which must both be present to achieve outcome benefits. A meta-analysis comprised of 35 trials was conducted by Drover et al [58], who reported that administering an arginine and fish oil-containing formula postoperatively reduced infection (RR 0.78; 95% CI, 0.64 to 0.95; P = 0.01) and hospital length of stay (WMD –2.23, 95% CI, –3.80 to –0.65; P = 0.006) but not mortality, when compared to use of a standard formula. Similar results were reported in a second meta-analysis [56], thus providing supportive evidence for the current SCCM/ASPEN recommendation to use an immune-modulating formula (containing both arginine and fish oils) in the SICU for the postoperative patient who requires EN therapy [12].
Safe, Consistent, and Effective Nutrition Support Therapy
Gastric vs Small Bowel Feeding
There is a large group of critically ill patients in whom impaired gastric emptying presents challenges to feeding; 50% of mechanically ventilated patients demonstrate delayed gastric emptying, and 80% of patients with increased intracranial pressure following head injury [60]. In one prospective RCT, Huang et al (2012) showed that severely ill patients (defined by an APACHE II score > 20) fed by the nasoduodenal route experienced significantly shortened hospital LOS, fewer complications, and improved nutrient delivery compared to similar patients fed by the nasogastric route. Less severely ill patients (APACHE II < 20) showed no differences between nasogastric and nasoduodenal feeding for the same outcomes [61]. A recent systematic review [17] pooled data from 14 trials of 1109 participants who received either gastric or small bowel feeding. Moderate quality evidence suggested that post-pyloric feeding was associated with lower rates of pneumonia compared with gastric feeding (RR 0.65, 95% CI, 0.51 to 0.84). Low-quality evidence showed an increase in the percentage of total nutrient delivered to the patient by post-pyloric feeding (mean difference 7.8%, 95% CI, 1.43 to 14.18). Overall, the authors found a 30% lower rate of pneumonia associated with post-pyloric feeding. There is insufficient evidence to show that other clinically important outcomes such as duration of mechanical ventilation, mortality, or LOS were affected by the site of feeding. The American Thoracic Society and ASPEN, as well as the Infectious Diseases Society of America, have published guidelines in support of small bowel feeding in the ICU setting due to its association with reduced incidence of health care–associated infections, specifically ventilator-associated pneumonia [62]. The experts who developed the SCCM/ASPEN and Canadian guidelines stress that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access [12,21]. The reality in ICU clinical practice is that many centers will begin with gastric feeding, barring absolute contraindications, and carefully monitor the patient for signs of intolerance before moving the feeding tube tip into a post-pyloric location. This follows the general recommendation by experts saying in most critically ill patients, it is acceptable to initiate EN in the stomach [12,21]. Protocols that guide management of risk prevention and intolerance typically recommend head of bed elevation, prokinetic agents, and frequent abdominal assessments [63,64].
Once the decision is made to use a post-pyloric feeding tube for nutrition therapy, the next decision is how to safely place the tube, ensure the tip is in an acceptable small bowel location, and minimize delays in feeding. Challenges related to feeding tube insertion may preclude timely advancement to nutrition goals. Placement of feeding tubes into the post-pyloric position is often done at the bedside by trained nursing or medical staff without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without risks. Success rates of this approach vary greatly depending on the patient population and provider expertise. Placement using endoscopic or fluoroscopic guidance is a safe alternative but usually requires coordinating a transport to the radiologic suite, posing safety risks and possible feeding delays for the patient [65]. Bedside use of an electromagnetic placement device (EMPD), such as Cortrak, provides yet another alternative with reports in the literature of 98% success rates for initial placement in less than 20 minutes. In a multicenter prospective study by Powers et al (2011), only one of 194 patients enrolled had data showing a discrepancy between the original EMPD verification and the final radiograph interpretation, demonstrating a 99.5% agreement between the two readings [20]. Median placement time was 12 minutes and no patient experienced an adverse event related to tube insertion using this device. The ability to monitor the location of the feeding tube tip in real time provides a desirable safety feature for the clinician performing bedside insertions. Nurses should consider incorporating the EMPD into the unit feeding protocol, as this would reduce the time to initiation of feedings with early and accurate tube insertion. Ongoing staff education and experience with the procedure are necessary elements to achieve the high rates of success often reported in the literature [66,67]. Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%, with complication rates of 1% to 3% for inadvertent placement of the feeding tube in the airway alone [65]. Radiographic confirmation of tube placement is advised prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.
Gastric Residual Volume Monitoring
A number of factors impede the delivery of EN in the critical care setting; these include gastrointestinal intolerance, under-prescribing to meet daily requirements, frequent interruptions for procedures, and technical issues with tube placement and maintaining patency [68]. Monitoring gastric residual volumes (GRV) contributes to these factors, yet volumes do not correlate well with incidence of pneumonia [69], measures of gastric emptying, or to the incidence of regurgitation and aspiration [70,71]. However, few studies have highlighted the difficulty of obtaining an accurate GRV due to feeding tube tip location, patient position, and type of tube [69]. Several high quality studies have demonstrated that raising the cutoff value for GRV from a lower number of 50–150 mL to a higher number of 250–500 mL does not increase risk for regurgitation, aspiration, or pneumonia [70,71]. A lower cutoff value for GRV does not protect the patient from complications, often leads to inappropriate cessation, and may adversely affect outcome through reduced volume of EN infused [72]. Gastric residual volumes in the range of 200–500 mL should raise concern and lead to the implementation of measures to reduce risk of aspiration, but automatic cessation of feeding should not occur for GRV < 500 mL in the absence of other signs of intolerance [12,69]. Metheny et al (2012) conducted a survey in which more than 97% of nurses responded that they assessed intolerance by measuring GRV; the most frequently cited threshold levels for interrupting feedings were 200 mL and 250 mL [73]. While threshold levels varied widely, only 12.6% of the nurse respondents reported allowing GRV up to 500 mL before interrupting feedings. While monitoring GRV is unnecessary with small bowel feeding, the location of the feeding tube tip should be questioned if gastric contents are obtained from a small bowel tube. The use of GRV as a parameter for trending may also yield important information regarding tolerance of feeding when the patient is unable to communicate abdominal discomfort. Other objective measures to use in the assessment of tolerance include an abdominal exam with documentation of changes in bowel sounds, expanding girth, tenderness or firmness on palpation, increasing nasogastric output, and vomiting [12,68]. If there are indications of intolerance, it is appropriate to divert the tip of the feeding tube into the distal small bowel as discussed previously.
Trophic vs Full Feeding
For the patient with low nutrition risk, there is a lack of convincing data to support an aggressive approach to feeding, either EN or PN, in the first week of critical illness [7]. In recent years, results of several trials suggest early goal-directed feeding in this population may cause net harm with increased morbidity and mortality. When discussing recent controversies in critical care nutrition, one must mention the two schools of thought when it comes to full versus limited energy provision in the first week following ICU admission. Studies in animals and humans have shown a trophic effect of enteral nutrients on the integrity of the gut mucosa, a finding that has provided the rationale for instituting enteral nutrition early during critical illness [15]. However, the inability to provide enteral nutrition early may be a marker of the severity of illness (ie, patients who can be fed enterally are less ill than those who cannot) rather than a mediator of complications and poor outcomes. Compher et al (2017) stated that greater nutritional intake is associated with lower mortality and faster time to discharge alive in high-risk, chronic patients but does not seem to be significant in nutritionally low-risk patients [74]. The findings of the EPaNIC and EDEN trials raised concern that targeting goals that meet full energy needs early in critical illness does not provide benefit and may cause harm in some populations or settings [32,75]. The EDEN trial [32] left us believing that trophic feeding at 10–20 mL/hr may be just as effective as any feeding in the first few days of critical illness striving for 15% to 20% of daily goal calories. After establishing tolerance, advancing daily intake to > 50% to 65% of goal calories, and up to 80% for the highest risk patients, may be required to prevent intestinal permeability and achieve positive clinical outcomes [33].
The systematic review and meta-analysis performed by Al-Dorzi et al (2016) adds further evidence for judicious advancement of EN for critically ill patients [76]. The authors reported finding no association between the dose of caloric intake and hospital mortality. Furthermore, a lower caloric intake resulted in lower risk of bloodstream infections and the need for renal replacement therapy (in 5 of the 21 trials only). As with many other meta-analyses, the authors reported that their results are most assuredly impacted by the heterogeneity in design, feeding route, and dose prescribed and delivered [16,76,77]. Other recent trials such as Arabi et al (2015) that enrolled 894 patients with different feeding targets further confirmed that there is no difference in outcome between groups when it comes to moderate (40% to 60% of goal) vs high (70% to 100% of goal) energy intake, infection rates, or 90-day mortality. The authors summarized their findings saying feeding closer to target is associated with better outcomes compared with severe underfeeding [78]. This adds to the controversy when considering the findings of still other RCTs or meta-analyses that evaluated minimal or trophic feeding versus standard feeding rates [9,46,77]. The meta-analysis performed by Marik and Hooper concluded that there were no differences in the risk of acquired infections, hospital mortality, ICU LOS, or ventilator-free days whether patients received intentional hypocaloric or normocaloric nutrition support [9]. Similarly, there was no significant difference in overall mortality between the underfeeding and full-feeding groups (OR, 0.94; 95% CI, 0.74–1.19; I2 = 26.6%; P = 0.61) in the meta-analysis done by Choi et al (2015), although only 4 trials were included to ensure homogeneity of the population and the intervention [77]. Furthermore, the hospital LOS and ICU LOS did not differ between the 2 groups, nor did any other secondary clinical outcome, leading the authors to conclude that calorie intake of the initial EN support for ICU patients had no bearing on relevant outcomes.
Recent studies have attempted to correlate caloric intake and patient outcomes without success; achieving 100% of caloric goal has not favorably impacted morbidity and mortality. Evidence suggests that intake greater than 65% to 70% of daily caloric requirement in the first 7 to 10 days of ICU stay may be associated with poorer outcomes, particularly when parenteral nutrition is used to supplement intake to achieve the caloric target [33–35].
Conclusion
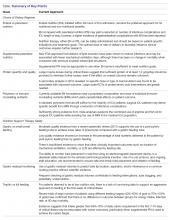
In this review we described current ICU controversies surrounding nutrition therapy and briefly discussed the data that support more than one course of action. A summary of key points covered is presented in the Table.
Disclaimer: The views expressed in this paper are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government.
Corresponding author: Mary S. McCarthy, PhD, RN, CNSC, 1611 Nisqually St, Steilacoom, WA 98388.
Financial disclosures: None.
1. Hippocratic Oath. Translated by Michael North, National Library of Medicine, 2002.
2. Nightingale F. Notes on Nursing. What it is and what it is not. Radford, VA: Wilder Publications, LLC;2007
3. White JV, Guenter P, Jensen G, et al; the Academy Malnutrition Work Group; the ASPEN Malnutrition Task Force; and the ASPEN Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–83.
4. Hooper MH, Marik PE. Controversies and misconceptions in Intensive Care Unit nutrition. Clin Chest Med 2015;36:409–18.
5. Patel JJ, Codner P. Controversies in critical care nutrition support. Crit Care Clin 2016;32:173–89.
6. Rosenthal MD, Vanzant EL, Martindale RG, Moore FA. Evolving paradigms in the nutritional support of critically ill surgical patients. Curr Probl Surg 2015;52:147–82.
7. McCarthy MS, Warren M, Roberts PR. Recent critical care nutrition trials and the revised guidelines: do they reconcile? Nutr Clin Pract 2016;31:150–4.
8. Barker LA, Gray C, Wilson L, et al. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr 2013;67: 802–807.
9. Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 2016;42:316–323.
10. Patkova A, Joskova V, Havel E, et al. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr 2017;8:624–34.
11. Wong CS, Aly EH. The effects of enteral immunonutrition in upper gastrointestinal surgery: a systematic review and meta-analysis. Int J Surg 2016;29:137–50.
12. McClave SA, Taylor BE, Martindale RG, et al; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society of Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr 2016; 40:159–211.
13. Ammori BJ. Importance of the early increase in intestinal permeability in critically ill patients. Eur J Surg 2002;168:660–1.
14. Vazquez-Sandoval A, Ghamande S, Surani S. Critically ill patients and gut motility: are we addressing it? World J Gastrointest Pharmacol Ther 2017;8:174–9.
15. Patel JJ, Martindale RG, McClave SA. Controversies surrounding critical care nutrition: an appraisal of permissive underfeeding, protein, and outcomes. JPEN J Parenter Enteral Nutr 2017; 148607117721908.
16. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg 2014;219:1078–87.
17. Alkhawaja S, Martin C, Butler RJ, Gwadry-Sridhar F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database of Syst Rev 2015; CD008875.
18. Davies AR, Morrison SS, Bailey MJ, et al ; ENTERIC Study Investigators; ANZICS Clinical Trials Group. A multi-center randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med 2012;40:2342–8.
19. Hsu CW, Sun SF, Lin SL, et al. Duodenal versus gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med 2009;37:1866–72.
20. Powers J, Luebbehusen M, Spitzer T, et al. Verification of an electromagnetic placement device compared with abdominal radiograph to predict accuracy of feeding tube placement. JPEN J Parenter Enteral Nutr 2011;35:535–9.
21. Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract 2014; 29:29–43.22. Kreymann K, Berger M, Deutz N. et al; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006;25:210–23.
23. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Ent Nutr 2009;33:277–316.
24. McClave SA, Martindale RG, Rice TW, Heyland DK. Feeding the critically ill patient. Crit Care Med 2014;42:2600–10.
25. Tian F, Gao X, Wu C, et al. Initial energy supplementation in critically ill patients receiving enteral nutrition: a systematic review and meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr 2017;26:11–9.
26. Martindale RG, Warren M. Should enteral nutrition be started in the first week of critical illness? Curr Opin Clin Nutr Metab Care 2015;18:202–6.
27. McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract 2009;24:305–15.
28. Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr 2007;31:461–82.
29. Seron-Arbeloa C, Zamora-Elson M, Labarta-Monzon L, Mallor-Bonet T. Enteral nutrition in critical care. J Clin Med Res 2013;5:1-11.
30. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673–84.
31. Elke G, van Zanten AR, Lemieux M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117.
32. Reignier J, Boisramé-Helms J, Brisard L, et al. Enteral versus parenteral nutrition in ventilated adults with shock: a randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133–43.
33. Rice TW , Wheeler AP, Thompson BT et al;National Heart, Lung, and Blood Institute, Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307:795–803.
34. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R258.
35. Bost RB, Tjan DH, van Zanten AR. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Ann Intensive Care 2014;4:31.
36. Casaer MP, Mesotten D, Hermans G, et al. Early verus late parenteral nutrition in critically ill adults. N Eng J Med 2011;365:506–17.
37. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Eng J Med 2014;371:1673–84.
38. Manzanares W, Dhaliwal R, Jurewitsch B, et al. Parenteral fish oil lipid emulsions in the critically ill: A systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014;38:20–8.
39. Oshima T, Heidegger CP, Pichard C. Supplemental parenteral nutrition is the key to prevent energy deficits in critically ill patients. Nutr Clin Prac 2016;31:432–7.
40. Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care 2015;19:167.
41. Rooyackers O, Sundström Rehal M, Liebau F, et al. High protein intake without concerns? Crit Care 2017;21:106.
42. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600.
43. Heyland D, Muscedere J, Wischmeyer PE, et al; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97.
44. Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med 2008;34:1980–90.
45. Gadek JE, DeMichele SJ, Karlstad MD, et al; Enteral Nutrition in ARDS Study Group. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med 1999;27:1409–20.
46. Rice TW, Wheeler AP, Thompson BT, et al; NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306:1574–81.
47. Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006;34:1033–38.
48. Atkinson S, Sieffert E, Bihari D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy’s Hospital Intensive Care Group. Crit Care Med 1998;26:1164–72.
49. Galbán C, Montejo JC, Mesejo A, et al. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 2000;28:643–8.
50. Weimann A, Bastian L, Bischoff WE, et al. Influence of arginine, omega-3 fatty acids and nucleotide-supplemented enteral support on systemic inflammatory response syndrome and multiple organ failure in patients after severe trauma. Nutrition 1998;14:165–72.
51. van Bokhorst-De Van Der Schueren MA, Quak JJ, von Blomberg-van der Flier BM, et al. Effect of perioperative nutrition with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am J Clin Nutr 2001;73:323–32.
52. Cerantola Y, Hübner M, Grass F, et al. Immunonutrition in gastrointestinal surgery. Br J Surg 2011;98:37–48.
53. Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr 2010;34:378–86.
54. Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid–supplemented enteral nutrition vs. standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg 2012;99:346–55.
55. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg 2006;30:1592–604.
56. Pearce CB, Sadek SA, Walters AM, et al. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP 2006;7:361–71.
57. Mazaki T, Ishii Y, Murai I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: a multiple treatments meta-analysis. Ann Surg 2015;261:662–9.
58. van Zanten ARH, Sztark F, Kaisers UX, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high protein enteral nutrition and nosocomial infections in the ICU. JAMA 2014;312:514–24.
59. Drover JW, Dhaliwal R, Weitzel L, et al. Perioperative use of arginine supplemented diets: a systematic review of the evidence. J Am Coll Surg 2011;212:385–99.
60. Stupak D, Abdelsayed GG, Soloway GN. Motility disorders of the upper gastrointestinal tract in the intensive care unit: pathophysiology and contemporary management. J Clin Gastroenterol 2012;46:449–56.
61. Huang HH, Chang SJ, Hsu CW, et al. Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet 2012;112:1138–46.
62. American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416.
63. Heyland DK, Cahill NE, Dhaliwal R, et al. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr 2010;34:675–84.
64. Landzinski J, Kiser TH, Fish DN, et al. Gastric motility function in critically ill patients tolerant vs intolerant to gastric nutrition. JPEN J Parenter Enteral Nutr 2008;32:45–50.
65. de Aguilar-Nascimento JE, Kudsk KA. Use of small bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care 2007;10:291–6.
66. Boyer N, McCarthy MS, Mount CA. Analysis of an electromagnetic tube placement device vs a self-advancing nasal jejunal device for postpyloric feeding tube placement . J Hosp Med 2014;9:23–8.
67. Metheny NA, Meert KL. Effectiveness of an electromagnetic feeding tube placement device in detecting inadvertent respiratory placement. Am J Crit Care 2014;23:240–8.
68. Montejo JC, Miñambres E, Bordejé L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 2010;36:1386–93.
69. Hurt RT, McClave SA. Gastric residual volumes in critical illness: what do they really mean? Crit Care Clin 2010;26:481–90.
70. Poulard F, Dimet J, Martin-Lefevre L, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr 2010;34:125–30.
71. Reignier J, Mercier E, Gouge AL, et al; Clinical Research in Intensive Care and Sepsis (CRICS) Group. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013;309:249–56.
72. Williams TA, Leslie GD, Leen T, et al. Reducing interruptions to continuous enteral nutrition in the intensive care unit: a comparative study. J Clin Nurs 2013;22:2838-2848.
73. Metheny NA, Stewart BJ, Mills AC. Blind insertion of feeding tubes in intensive care units: a national survey. Am J Crit Care 2012;21:352–360.
74. Compher C, Chittams J, Sammarco T, et al. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: A multicenter, multinational observational study. Crit Care Med 2017;45:156–163.
75. Casaer MP, Wilmer A, Hermans G, et al. Role of disease and macronutrient dose in the randomized controlled EPANIC Trial: a post hoc analysis. Am J Resp Crit Care Med 2013;187:247–55.
76. Al-Dorzi HM, Albarrak A, Ferwana M, et al. Lower versus higher dose of enteral caloric intake in adult critically ill patients: a systematic review and meta-analysis. Crit Care 2016;20:358.
77. Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2015;39:291–300.
78. Arabi YM, Aldawood AS, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med 2015;372:2398–408.
From the Center for Nursing Science & Clinical Inquiry (Dr. McCarthy), and Nutrition Care Division, (Ms. Phipps), Madigan Army Medical Center, Tacoma, WA.
Abstract
- Background: Many controversies exist in the field of nutrition support today, particularly in the critical care environment where nutrition plays a more primary rather than adjunctive role.
- Objective: To provide a brief review of current controversies regarding nutrition therapy in the ICU focusing on the choices regarding the nutrition regimen and the safe, consistent delivery of nutrition as measured by clinical outcomes.
- Methods: Selected areas of controversy are discussed detailing the strengths and weaknesses of the research behind opposing opinions.
- Results: ICU nutrition support controversies include enteral vs parenteral nutrition, use of supplmental parenteral nutrition, protein quantity and quality, and polymeric vs immune-modulating nutrients. Issues surrounding the safety of nutrition support therapy include gastric vs small bowel feeding and trophic vs full feeding. Evidence-based recommendations published by professional societies are presented.
- Conclusion: Understanding a patient’s risk for disease and predicting their response to treatment will assist clinicians with selecting those nutrition interventions that will achieve the best possible outcomes.
According to the National Library of Medicine’s translation of the Hippocratic oath, nowhere does it explicitly say “First, do no harm.” What is written is this: “I will use those dietary regimens which will benefit my patients according to my greatest ability and judgement, and I will do no harm or injustice to them” [1]. In another renowned text, one can find this observation regarding diet by a noted scholar, clinician, and the founder of modern nursing, Florence Nightingale: “Every careful observer of the sick will agree in this that thousands of patients are annually starved in the midst of plenty, from want of attention to the ways which alone make it possible for them to take food” [2,]. While Nightingale was alluding to malnutrition of hospitalized patients, it seems that her real concern may have been the iatrogenic malnutrition that inevitably accompanies hospitalization, even today [3].
From these philosophic texts, we have two ongoing controversies in modern day nutrition therapy identified: (1) what evidence do we have to support the choice of dietary regimens (ie, enteral vs parenteral therapy, timing of supplemental parenteral nutrition, standard vs high protein formula, polymeric vs immune-modulating nutrients) that best serve critically ill patients, and (2) how do we ensure that ICU patients are fed in a safe, consistent, and effective manner (gastric vs small bowel tube placement, gastric residual monitoring or not, trophic vs full feeding) as measured by clinically relevant outcomes? Many controversies exist in the field of nutrition support today [4–7] and a comprehensive discussion of all of them is beyond the scope of this paper. In this paper we will provide a brief review of current controversies focusing on those mentioned above which have only recently been challenged by new rigorous randomized clinical trials (RCTs), and in some cases, subsequent systematic reviews and meta-analyses [8–11].
The Path to Modern Day Nutrition Support Therapy
The field of nutrition support, in general, has expanded greatly over the last 4 decades, but perhaps the most notable advancements have occurred in the critical care environment where efforts have been directed at advancing our understanding of the molecular and biological effects of nutrients in maintaining homeostasis in the critically ill [6]. In recent years, specialized nutrition, delivered by the enteral or parenteral route, was finally recognized for its contribution to important clinical outcomes in the critically ill population [12]. Critical care clinicians have been educated about the advances in nutrition therapy designed to address the unique needs of a vulnerable population where survival is threatened by poor nutritional status upon admission, compromised immune function, weakened respiratory muscles with decreased ventilation capacity, and gastrointestinal (GI) dysfunction [6]. The rapid deterioration seen in these patients is exaggerated by the all too common ICU conditions of systemic inflammatory response syndrome (SIRS), sepsis, hemodynamic instability, respiratory failure, coagulation disorders, and acute kidney injury [13,14].
Beginning in the early 1990s, formulations of enteral nutrition (EN) contained active nutrients that reportedly reduced oxidative damage to cells and tissues, modulated inflammation, and improved feeding tolerance. These benefits are now referred to as the non-nutritive benefits of enteral feeding [15]. For the next 20 years, scientific publications released new results from studies examining the role of omega-3 fatty acids, antioxidant vitamins, minerals such as selenium and zinc, ribonucleotides, and conditionally essential amino acids like glutamine and arginine, in healing and recovery from critical illness. The excitement was summarized succinctly by Hegazi and Wischmeyer in 2011 when they remarked that the modern ICU clinician now has scientific data to guide specialized nutrition therapy, for example, choosing formulas supplemented with anti-inflammatory, immune-modulating, or tolerance-promoting nutrients that have the potential to enhance natural recovery processes and prevent complications [16].
The improvements in nutritional formulas were accompanied by numerous technological advances including bedside devices (electromagnetic enteral access system, real-time image-guided disposable feeding tube, smart feeding pumps with water flush technology) that quickly and safely establish access for small bowel feedings, which help minimize risk of gastric aspiration and ventilator-associated pneumonia, promote tolerance, decrease radiologic exposure, and may reduce nursing time consumed by tube placements, GI dysfunction, and patient discomfort [17–20]. Nasogastric feeding remains the most common first approach, with local practices, contraindications, and ease of placement usually determining the location of the feeding tube [5]. The advancements helped to overcome the many barriers to initiating and maintaining feedings and thus, efforts to feed critically ill patients early and effectively became more routine, along with nurse, patient, and family satisfaction. In conjunction with the innovative approaches to establishing nutrition therapy, practice guidelines published by United States, European, and Canadian nutrition societies became widely available in the past decade with graded evidence-based recommendations for who, when, what, and how to feed, and unique considerations for various critically ill populations [12,21,22]. The tireless efforts by the nutrition societies to provide much needed guidelines for clinicians were appreciated, yet there was a wide range in the grade of the recommendations, with many based on expert opinion alone. In some cases, the research conducted lacked rigor or had missing data with obvious limits to the generalizability of results. Nevertheless, for the 7 years between the publication of the old and newly revised Society of Critical Care Medicine (SCCM)/ American Society of Parenteral and Enteral Nutrition (ASPEN) Guidelines (2016), [12,23] nutrition therapy was a high-priority intervention in most ICUs. The goal was to initiate feeding within 48 hours, select an immune-modulating or other metabolic support formula, and aggressively advance the rate to 80% to 100% of goal to avoid caloric deficit, impaired intestinal integrity, nitrogen losses, and functional impairments [9,24,25]. Nutrition support evolved from adjunctive care to its rightful place in the ABCD mnemonic of early priorities of ICU care: Airway, Breathing, Circulation, Diet.
The 2016 joint publication of the SCCM/ASPEN guidelines includes primarily randomized controlled trial (RCT) data, along with some observational trial data, indexed in any major publication database through December 2013. In these guidelines there were 98 recommendations, of which only 5 were a Level 1A; most of the recommendations were categorized as “expert consensus” [12]. The results of several important clinical trials in the United States and Europe that were underway at the time have since been published and compared to the SCCM/ASPEN relevant recommendations [7]. The results have forced nutrition support clinicians to take a step back and re-examine their practice. For many seasoned clinicians who comprised the nutrition support teams of the 1980s and 1990s, it feels like a return to the basics. Until biology-driven personalized medicine is commonplace and genotype data is readily available to guide nutrition therapy for each critically ill patient, standard enteral feeding that begins slow and proceeds carefully over 5 to 7 days towards 80% of goal caloric intake under judicious monitoring of biochemical and metabolic indices may be the “best practice” today, without risk of harm [15,26]. As in all aspects of clinical care, this practice is not without controversy.
ICU Nutrition Support Controversies Today
Enteral vs Parenteral Nutrition
There is universal consensus that EN is the preferred route for nutrition therapy due to the superior physiological response and both nutritional and non-nutritional benefits [24]. Changes in gut permeability tend to occur as illness progresses and consequences include increased bacterial challenge, risk for multiple organ dysfunction syndrome, and systemic infection. It is best to intervene with nutrition early, defined as within the first 48 hours of ICU admission, while the likelihood of success and opportunity to impact the disease process is greater [12]. Early initiation of feeding provides the necessary nutrients to support gut-associated lymphoid tissue (GALT), mucosal-associated lymphoid tissue (MALT), and preserve gut integrity and microbial diversity [27]. The intestine is an effective barrier against bacteria and intraluminal toxins due to the high rate of enterocyte turnover, the mucus secreted by the goblet cells, and the large amount of protective immunological tissue; 80% of the immunoglobulins are synthesized in the GI tract [28]. Fasting states for procedures or delays in feeding longer than 3 days for any reason may contribute to disruption of intestinal integrity through atrophy and derangements in the physical structure and function of the microvilli and crypts [29]. Intestinal dysfunction leads to increased intestinal permeability and the possibility of bacterial translocation. Intestinal ischemia resulting from shock and sepsis may produce hypoxia and reperfusion injuries further affecting intestinal wall permeability [29]. In surgical patients, early enteral feeding has been found to reduce inflammation, oxidative stress, and the catabolic response to anesthesia and surgical-induced stress, help restore intestinal motility, reverse enteric mucosal atrophy, and improve wound healing [26].
We did not have sufficient data to refute the benefits of EN over PN until the paper by Harvey et al (2014), which reported no difference in mortality or infectious complications in ICU patients receiving EN or PN within 36 hours of admission and for up to 5 days [30]. This was the largest published pragmatic RCT, referred to as the CALORIES trial, which analyzed 2388 patients from 33 ICUs and resulted in controversy over what was an unchallenged approach up until this time. It was only a matter of time before other investigators would set out to confirm or negate this finding, which is what Elke and coworkers (2016) did a few years later [31]. They performed an updated systematic review and meta-analysis to evaluate the overall effect of the route of nutrition (EN versus PN) on clinical outcomes in adult critically ill patients. Similar to the Harvey et al report, they found no difference in mortality between the two routes of nutrition. However, unlike the earlier report, patients receiving EN compared to PN had a significant reduction in the number of infectious complications and ICU length of stay. No significant effect was found for hospital length of stay or days requiring mechanical ventilation. The authors suggest that EN delivery of macronutrients below predefined targets may be responsible as PN is more likely to meet or exceed these targets and overwhelm metabolic capacity in the early days of critical illness [31].
The most recent trial prompting even more discussion about early PN versus early EN in mechanically ventilated ICU patients in shock is the Reignier et al (2018) NUTRIREA-2 trial involving 2410 patients from 44 ICUs in France [32]. The investigators hypothesized that outcomes would be better with early exclusive EN compared to early exclusive PN; their hypothesis was not supported by the results, which found no difference in 28-day mortality or ICU-acquired infections. Also unexpected was the higher cumulative incidence of gastrointestinal complications including vomiting, diarrhea, bowel ischemia, and acute colonic obstruction in the EN group. The trial was stopped early after an interim analysis determined that additional enrollment was not likely to significantly change the results of the trial. Given the similarities between the CALORIES trial and this NUTRIREA-2 trial, clinicians now have mounting evidence that equivalent options for nutrition therapy exist and an appropriate selection should be made based on patient-specific indications and treatment goals. In summary, EN remains preferable to PN for the majority of adult critically ill patients due to crucial support of gut integrity, but the optimal dose or rate of delivery to favorably influence clinical outcomes in the first few days following admission remains unknown.
Use of Supplemental Parenteral Nutrition
Both the nutrition support and bench science communities have learned a great deal about PN over the 4 decades it has been in existence, with the most compelling data coming from more recent trials [31–38]. This is because it has taken many years to recover from the days of hyperalimentation or overfeeding ICU patients by providing excessive calories to meet the elevated energy demands and to reverse the hypercatabolism of critical illness. This approach contributed to the complications of hyperglycemia, hyperlipidemia, increased infectious complications, and liver steatosis, all of which gave PN a negative reputation [37]. We now have adjusted the caloric distribution and the actual formulation of PN using the recent FDA-approved lipid emulsion (Soy, Medium-chain triglycerides, Olive oil, and Fish oil; SMOF) and created protocols for administering it based on specific indications, such as loss of GI integrity or demonstrated intolerance. In general, the advances in PN have led to a safer more therapeutic formulation that has its place in critical illness. Manzanares et al [40] reported a trend toward a decrease in ventilation requirement and mortality when a fish oil–containing lipid emulsion was administered to patients who were receiving nutrition support either enterally or parenterally. The meta-analysis combined all soybean oil–sparing lipid emulsions for comparison with soybean oil and was able to show the trend for improved clinical outcomes with soybean oil–sparing lipid emulsions. The main findings of this meta-analysis were that fish oil–containing lipid emulsions may reduce infections and may be associated with a tendency toward fewer mechanical ventilation days, although not mortality, when compared with soybean oil-based strategies or administration of other alternative lipid emulsions in ICU patients [40]. Recent trial results do not change the recommendation for selecting EN first but do suggest lowering the threshold for using PN when EN alone is insufficient to meet nutrition goals. A systematic review reported no benefit of early supplemental PN over late supplemental PN and cautioned that our continued inability to explain greater infectious morbidity and unresolved organ failure limits any justification for early PN [35].
Protein Quantity and Quality
The practice of providing protein in the range of 1.2–2.0 g/kg actual body weight early in critical illness is suggested by expert consensus in the 2016 SCCM/ASPEN guidelines [12]; however, the evidence for efficacy remains controversial. It is argued that the evidence for benefit comes from observational studies, not from prospective RCTs, and that the patients at high risk are often excluded from study protocols. It has also been suggested that in patients with limited vital organ function increased protein delivery may lead to organ compromise. In a recent (2017) paper, Rooyackers et al discussed the post-hoc analyses of data from the EPANIC Trial stating the statistical correlation between protein intake and outcomes indicate that protein was associated with unfavorable outcomes, possibly by inhibiting autophagy [41].
The nutrition support community may have widely varying approaches to feeding critically ill patients but most experts agree that protein may be the most important macronutrient delivered during critical illness. There is consensus that the hypercatabolism associated with stress induces proteolysis and the loss of lean muscle mass, which may affect clinical and functional outcomes beyond the ICU stay. Using multiple assessment modalities, Puthucheary et al (2013) demonstrated a reduction in the rectus femoris muscle of 12.5% over the first week of hospitalization in the ICU and up to 17.7% by day 10. These numbers imply that sufficient protein of at least 1.2 g/kg/day should be provided to minimize these losses, even if the effect on overall outcome remains unknown [42]. Evidence is lacking for whether or not we can prevent the muscle wasting that occurs in critical illness with increased protein dosing. We also need to better identify the possible risks involved with a high-protein intake at the level of the individual patient. A secondary analysis done by Heyland et al (2013) determined that no specific dose or type of macronutrient was found to be associated with improved outcome [43]. It is clear that more large-scale RCTs of protein/amino acid interventions are needed to prove that these nutrition interventions have favorable effects on clinically important outcomes, including long-term physical function.
Polymeric vs Immune-Modulating Nutrients
The Marik and Zaloga (2008) systematic review on immunonutrition in the critically ill convinced most clinicians that while fish-oil based immunonutrition improves the outcome of medical ICU patients, diets supplemented with arginine, with or without glutamine or fish oils, do not demonstrate an advantage over standard enteral products in general ICU, trauma, or burn patients[44]. What followed these trials examining early formulations of immunonutrition was decades of well-intentioned research dedicated to elucidating the mechanism of action for individual immune-modulating nutrients for various populations, including those with acute lung injury/acute respiratory distress syndrome (ARDS) [45–47], sepsis/systemic inflammatory response syndrome [48–50], head and neck cancer [51], upper and lower GI cancer [52–55], and severe acute pancreatitis [56]. Our understanding of immunonutrition and the administration of this formulation in specific disease conditions has grown considerably yet clinicians are still asking exactly what is the role of immunonutrition and who stands to benefit the most from immune-modulating nutrition therapy. The enteral formulations currently available have a proprietary composition and dosage of individual nutrients which yield unpredictable physiologic effects. In addition, the pervasive problem of underfeeding during hospitalization prevents adequate delivery of physiologic doses of nutrients thus contributing to the widely variable research results.
Prevailing expert opinion today is that the majority of critically ill patients will benefit from nutrition support initiated early and delivered consistently; the standard polymeric formula will suffice for the majority of patients with surgical ICU patients potentially deriving benefit from immunonutrition that supports a reduction in infectious complications [57]. In the recent multiple-treatment meta-analysis performed by Mazaki et al (2015) involving 74 studies and 7572 patients, immunonutrition was ranked first for reducing the incidence of 7 complications according to the surface under the cumulative ranking curve; these were as follows: any infection, 0.86; overall complication, 0.88; mortality, 0.81; wound infection, 0.79; intra-abdominal abscess, 0.98; anastomotic leak, 0.79; and sepsis, 0.92. immunonutrition was ranked second for reducing ventilator-associated pneumonia and catheter-associated urinary tract infection (CAUTI), behind immune-modulating PN. The authors stated that immunonutrition was efficacious for reducing the incidence of complications in GI surgery unrelated to the timing of administration [57]. The 2014 publication of results from the MetaPlus Trial [58]challenged the published recommendations for the use of immunonutrition in the medical critically ill population. This trial used high-protein immunonutrition or standard formula for 301 adult critically ill patients in 14 European ICUs with diagnoses such as pneumonia or infections of the urinary tract, bloodstream, central nervous system, eye, ear, nose or throat, and the skin and soft tissue. Even with higher than average target energy intakes of 70% for the high protein immunonutrition group and 80% for the high protein standard group, there were no statistically significant differences in the primary outcome of new infections, or the secondary outcomes of days on mechanical ventilation, Sequential Organ Failure Assessment scores, or ICU and hospital length of stay. However, the 6-month mortality rate of 28% was higher in the medical subgroup [58]. Using these results, as well as existing publications of negative outcomes in medical ICU patients [44,46], the SCCM/ASPEN Guidelines Committee updated its position in 2016 to suggest that immunonutrition formulations or disease-specific formulations should no longer be used routinely in medical ICU patients, including those with acute lung injury/ARDS [12]. The Committee did suggest that these formulations should be reserved for patients with traumatic brain injury and for surgical ICU patients. The benefit for ICU postoperative patients has been linked to the synergistic effect of fish oil and arginine, which must both be present to achieve outcome benefits. A meta-analysis comprised of 35 trials was conducted by Drover et al [58], who reported that administering an arginine and fish oil-containing formula postoperatively reduced infection (RR 0.78; 95% CI, 0.64 to 0.95; P = 0.01) and hospital length of stay (WMD –2.23, 95% CI, –3.80 to –0.65; P = 0.006) but not mortality, when compared to use of a standard formula. Similar results were reported in a second meta-analysis [56], thus providing supportive evidence for the current SCCM/ASPEN recommendation to use an immune-modulating formula (containing both arginine and fish oils) in the SICU for the postoperative patient who requires EN therapy [12].
Safe, Consistent, and Effective Nutrition Support Therapy
Gastric vs Small Bowel Feeding
There is a large group of critically ill patients in whom impaired gastric emptying presents challenges to feeding; 50% of mechanically ventilated patients demonstrate delayed gastric emptying, and 80% of patients with increased intracranial pressure following head injury [60]. In one prospective RCT, Huang et al (2012) showed that severely ill patients (defined by an APACHE II score > 20) fed by the nasoduodenal route experienced significantly shortened hospital LOS, fewer complications, and improved nutrient delivery compared to similar patients fed by the nasogastric route. Less severely ill patients (APACHE II < 20) showed no differences between nasogastric and nasoduodenal feeding for the same outcomes [61]. A recent systematic review [17] pooled data from 14 trials of 1109 participants who received either gastric or small bowel feeding. Moderate quality evidence suggested that post-pyloric feeding was associated with lower rates of pneumonia compared with gastric feeding (RR 0.65, 95% CI, 0.51 to 0.84). Low-quality evidence showed an increase in the percentage of total nutrient delivered to the patient by post-pyloric feeding (mean difference 7.8%, 95% CI, 1.43 to 14.18). Overall, the authors found a 30% lower rate of pneumonia associated with post-pyloric feeding. There is insufficient evidence to show that other clinically important outcomes such as duration of mechanical ventilation, mortality, or LOS were affected by the site of feeding. The American Thoracic Society and ASPEN, as well as the Infectious Diseases Society of America, have published guidelines in support of small bowel feeding in the ICU setting due to its association with reduced incidence of health care–associated infections, specifically ventilator-associated pneumonia [62]. The experts who developed the SCCM/ASPEN and Canadian guidelines stress that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access [12,21]. The reality in ICU clinical practice is that many centers will begin with gastric feeding, barring absolute contraindications, and carefully monitor the patient for signs of intolerance before moving the feeding tube tip into a post-pyloric location. This follows the general recommendation by experts saying in most critically ill patients, it is acceptable to initiate EN in the stomach [12,21]. Protocols that guide management of risk prevention and intolerance typically recommend head of bed elevation, prokinetic agents, and frequent abdominal assessments [63,64].
Once the decision is made to use a post-pyloric feeding tube for nutrition therapy, the next decision is how to safely place the tube, ensure the tip is in an acceptable small bowel location, and minimize delays in feeding. Challenges related to feeding tube insertion may preclude timely advancement to nutrition goals. Placement of feeding tubes into the post-pyloric position is often done at the bedside by trained nursing or medical staff without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without risks. Success rates of this approach vary greatly depending on the patient population and provider expertise. Placement using endoscopic or fluoroscopic guidance is a safe alternative but usually requires coordinating a transport to the radiologic suite, posing safety risks and possible feeding delays for the patient [65]. Bedside use of an electromagnetic placement device (EMPD), such as Cortrak, provides yet another alternative with reports in the literature of 98% success rates for initial placement in less than 20 minutes. In a multicenter prospective study by Powers et al (2011), only one of 194 patients enrolled had data showing a discrepancy between the original EMPD verification and the final radiograph interpretation, demonstrating a 99.5% agreement between the two readings [20]. Median placement time was 12 minutes and no patient experienced an adverse event related to tube insertion using this device. The ability to monitor the location of the feeding tube tip in real time provides a desirable safety feature for the clinician performing bedside insertions. Nurses should consider incorporating the EMPD into the unit feeding protocol, as this would reduce the time to initiation of feedings with early and accurate tube insertion. Ongoing staff education and experience with the procedure are necessary elements to achieve the high rates of success often reported in the literature [66,67]. Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%, with complication rates of 1% to 3% for inadvertent placement of the feeding tube in the airway alone [65]. Radiographic confirmation of tube placement is advised prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.
Gastric Residual Volume Monitoring
A number of factors impede the delivery of EN in the critical care setting; these include gastrointestinal intolerance, under-prescribing to meet daily requirements, frequent interruptions for procedures, and technical issues with tube placement and maintaining patency [68]. Monitoring gastric residual volumes (GRV) contributes to these factors, yet volumes do not correlate well with incidence of pneumonia [69], measures of gastric emptying, or to the incidence of regurgitation and aspiration [70,71]. However, few studies have highlighted the difficulty of obtaining an accurate GRV due to feeding tube tip location, patient position, and type of tube [69]. Several high quality studies have demonstrated that raising the cutoff value for GRV from a lower number of 50–150 mL to a higher number of 250–500 mL does not increase risk for regurgitation, aspiration, or pneumonia [70,71]. A lower cutoff value for GRV does not protect the patient from complications, often leads to inappropriate cessation, and may adversely affect outcome through reduced volume of EN infused [72]. Gastric residual volumes in the range of 200–500 mL should raise concern and lead to the implementation of measures to reduce risk of aspiration, but automatic cessation of feeding should not occur for GRV < 500 mL in the absence of other signs of intolerance [12,69]. Metheny et al (2012) conducted a survey in which more than 97% of nurses responded that they assessed intolerance by measuring GRV; the most frequently cited threshold levels for interrupting feedings were 200 mL and 250 mL [73]. While threshold levels varied widely, only 12.6% of the nurse respondents reported allowing GRV up to 500 mL before interrupting feedings. While monitoring GRV is unnecessary with small bowel feeding, the location of the feeding tube tip should be questioned if gastric contents are obtained from a small bowel tube. The use of GRV as a parameter for trending may also yield important information regarding tolerance of feeding when the patient is unable to communicate abdominal discomfort. Other objective measures to use in the assessment of tolerance include an abdominal exam with documentation of changes in bowel sounds, expanding girth, tenderness or firmness on palpation, increasing nasogastric output, and vomiting [12,68]. If there are indications of intolerance, it is appropriate to divert the tip of the feeding tube into the distal small bowel as discussed previously.
Trophic vs Full Feeding
For the patient with low nutrition risk, there is a lack of convincing data to support an aggressive approach to feeding, either EN or PN, in the first week of critical illness [7]. In recent years, results of several trials suggest early goal-directed feeding in this population may cause net harm with increased morbidity and mortality. When discussing recent controversies in critical care nutrition, one must mention the two schools of thought when it comes to full versus limited energy provision in the first week following ICU admission. Studies in animals and humans have shown a trophic effect of enteral nutrients on the integrity of the gut mucosa, a finding that has provided the rationale for instituting enteral nutrition early during critical illness [15]. However, the inability to provide enteral nutrition early may be a marker of the severity of illness (ie, patients who can be fed enterally are less ill than those who cannot) rather than a mediator of complications and poor outcomes. Compher et al (2017) stated that greater nutritional intake is associated with lower mortality and faster time to discharge alive in high-risk, chronic patients but does not seem to be significant in nutritionally low-risk patients [74]. The findings of the EPaNIC and EDEN trials raised concern that targeting goals that meet full energy needs early in critical illness does not provide benefit and may cause harm in some populations or settings [32,75]. The EDEN trial [32] left us believing that trophic feeding at 10–20 mL/hr may be just as effective as any feeding in the first few days of critical illness striving for 15% to 20% of daily goal calories. After establishing tolerance, advancing daily intake to > 50% to 65% of goal calories, and up to 80% for the highest risk patients, may be required to prevent intestinal permeability and achieve positive clinical outcomes [33].
The systematic review and meta-analysis performed by Al-Dorzi et al (2016) adds further evidence for judicious advancement of EN for critically ill patients [76]. The authors reported finding no association between the dose of caloric intake and hospital mortality. Furthermore, a lower caloric intake resulted in lower risk of bloodstream infections and the need for renal replacement therapy (in 5 of the 21 trials only). As with many other meta-analyses, the authors reported that their results are most assuredly impacted by the heterogeneity in design, feeding route, and dose prescribed and delivered [16,76,77]. Other recent trials such as Arabi et al (2015) that enrolled 894 patients with different feeding targets further confirmed that there is no difference in outcome between groups when it comes to moderate (40% to 60% of goal) vs high (70% to 100% of goal) energy intake, infection rates, or 90-day mortality. The authors summarized their findings saying feeding closer to target is associated with better outcomes compared with severe underfeeding [78]. This adds to the controversy when considering the findings of still other RCTs or meta-analyses that evaluated minimal or trophic feeding versus standard feeding rates [9,46,77]. The meta-analysis performed by Marik and Hooper concluded that there were no differences in the risk of acquired infections, hospital mortality, ICU LOS, or ventilator-free days whether patients received intentional hypocaloric or normocaloric nutrition support [9]. Similarly, there was no significant difference in overall mortality between the underfeeding and full-feeding groups (OR, 0.94; 95% CI, 0.74–1.19; I2 = 26.6%; P = 0.61) in the meta-analysis done by Choi et al (2015), although only 4 trials were included to ensure homogeneity of the population and the intervention [77]. Furthermore, the hospital LOS and ICU LOS did not differ between the 2 groups, nor did any other secondary clinical outcome, leading the authors to conclude that calorie intake of the initial EN support for ICU patients had no bearing on relevant outcomes.
Recent studies have attempted to correlate caloric intake and patient outcomes without success; achieving 100% of caloric goal has not favorably impacted morbidity and mortality. Evidence suggests that intake greater than 65% to 70% of daily caloric requirement in the first 7 to 10 days of ICU stay may be associated with poorer outcomes, particularly when parenteral nutrition is used to supplement intake to achieve the caloric target [33–35].
Conclusion
In this review we described current ICU controversies surrounding nutrition therapy and briefly discussed the data that support more than one course of action. A summary of key points covered is presented in the Table.
Disclaimer: The views expressed in this paper are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government.
Corresponding author: Mary S. McCarthy, PhD, RN, CNSC, 1611 Nisqually St, Steilacoom, WA 98388.
Financial disclosures: None.
From the Center for Nursing Science & Clinical Inquiry (Dr. McCarthy), and Nutrition Care Division, (Ms. Phipps), Madigan Army Medical Center, Tacoma, WA.
Abstract
- Background: Many controversies exist in the field of nutrition support today, particularly in the critical care environment where nutrition plays a more primary rather than adjunctive role.
- Objective: To provide a brief review of current controversies regarding nutrition therapy in the ICU focusing on the choices regarding the nutrition regimen and the safe, consistent delivery of nutrition as measured by clinical outcomes.
- Methods: Selected areas of controversy are discussed detailing the strengths and weaknesses of the research behind opposing opinions.
- Results: ICU nutrition support controversies include enteral vs parenteral nutrition, use of supplmental parenteral nutrition, protein quantity and quality, and polymeric vs immune-modulating nutrients. Issues surrounding the safety of nutrition support therapy include gastric vs small bowel feeding and trophic vs full feeding. Evidence-based recommendations published by professional societies are presented.
- Conclusion: Understanding a patient’s risk for disease and predicting their response to treatment will assist clinicians with selecting those nutrition interventions that will achieve the best possible outcomes.
According to the National Library of Medicine’s translation of the Hippocratic oath, nowhere does it explicitly say “First, do no harm.” What is written is this: “I will use those dietary regimens which will benefit my patients according to my greatest ability and judgement, and I will do no harm or injustice to them” [1]. In another renowned text, one can find this observation regarding diet by a noted scholar, clinician, and the founder of modern nursing, Florence Nightingale: “Every careful observer of the sick will agree in this that thousands of patients are annually starved in the midst of plenty, from want of attention to the ways which alone make it possible for them to take food” [2,]. While Nightingale was alluding to malnutrition of hospitalized patients, it seems that her real concern may have been the iatrogenic malnutrition that inevitably accompanies hospitalization, even today [3].
From these philosophic texts, we have two ongoing controversies in modern day nutrition therapy identified: (1) what evidence do we have to support the choice of dietary regimens (ie, enteral vs parenteral therapy, timing of supplemental parenteral nutrition, standard vs high protein formula, polymeric vs immune-modulating nutrients) that best serve critically ill patients, and (2) how do we ensure that ICU patients are fed in a safe, consistent, and effective manner (gastric vs small bowel tube placement, gastric residual monitoring or not, trophic vs full feeding) as measured by clinically relevant outcomes? Many controversies exist in the field of nutrition support today [4–7] and a comprehensive discussion of all of them is beyond the scope of this paper. In this paper we will provide a brief review of current controversies focusing on those mentioned above which have only recently been challenged by new rigorous randomized clinical trials (RCTs), and in some cases, subsequent systematic reviews and meta-analyses [8–11].
The Path to Modern Day Nutrition Support Therapy
The field of nutrition support, in general, has expanded greatly over the last 4 decades, but perhaps the most notable advancements have occurred in the critical care environment where efforts have been directed at advancing our understanding of the molecular and biological effects of nutrients in maintaining homeostasis in the critically ill [6]. In recent years, specialized nutrition, delivered by the enteral or parenteral route, was finally recognized for its contribution to important clinical outcomes in the critically ill population [12]. Critical care clinicians have been educated about the advances in nutrition therapy designed to address the unique needs of a vulnerable population where survival is threatened by poor nutritional status upon admission, compromised immune function, weakened respiratory muscles with decreased ventilation capacity, and gastrointestinal (GI) dysfunction [6]. The rapid deterioration seen in these patients is exaggerated by the all too common ICU conditions of systemic inflammatory response syndrome (SIRS), sepsis, hemodynamic instability, respiratory failure, coagulation disorders, and acute kidney injury [13,14].
Beginning in the early 1990s, formulations of enteral nutrition (EN) contained active nutrients that reportedly reduced oxidative damage to cells and tissues, modulated inflammation, and improved feeding tolerance. These benefits are now referred to as the non-nutritive benefits of enteral feeding [15]. For the next 20 years, scientific publications released new results from studies examining the role of omega-3 fatty acids, antioxidant vitamins, minerals such as selenium and zinc, ribonucleotides, and conditionally essential amino acids like glutamine and arginine, in healing and recovery from critical illness. The excitement was summarized succinctly by Hegazi and Wischmeyer in 2011 when they remarked that the modern ICU clinician now has scientific data to guide specialized nutrition therapy, for example, choosing formulas supplemented with anti-inflammatory, immune-modulating, or tolerance-promoting nutrients that have the potential to enhance natural recovery processes and prevent complications [16].
The improvements in nutritional formulas were accompanied by numerous technological advances including bedside devices (electromagnetic enteral access system, real-time image-guided disposable feeding tube, smart feeding pumps with water flush technology) that quickly and safely establish access for small bowel feedings, which help minimize risk of gastric aspiration and ventilator-associated pneumonia, promote tolerance, decrease radiologic exposure, and may reduce nursing time consumed by tube placements, GI dysfunction, and patient discomfort [17–20]. Nasogastric feeding remains the most common first approach, with local practices, contraindications, and ease of placement usually determining the location of the feeding tube [5]. The advancements helped to overcome the many barriers to initiating and maintaining feedings and thus, efforts to feed critically ill patients early and effectively became more routine, along with nurse, patient, and family satisfaction. In conjunction with the innovative approaches to establishing nutrition therapy, practice guidelines published by United States, European, and Canadian nutrition societies became widely available in the past decade with graded evidence-based recommendations for who, when, what, and how to feed, and unique considerations for various critically ill populations [12,21,22]. The tireless efforts by the nutrition societies to provide much needed guidelines for clinicians were appreciated, yet there was a wide range in the grade of the recommendations, with many based on expert opinion alone. In some cases, the research conducted lacked rigor or had missing data with obvious limits to the generalizability of results. Nevertheless, for the 7 years between the publication of the old and newly revised Society of Critical Care Medicine (SCCM)/ American Society of Parenteral and Enteral Nutrition (ASPEN) Guidelines (2016), [12,23] nutrition therapy was a high-priority intervention in most ICUs. The goal was to initiate feeding within 48 hours, select an immune-modulating or other metabolic support formula, and aggressively advance the rate to 80% to 100% of goal to avoid caloric deficit, impaired intestinal integrity, nitrogen losses, and functional impairments [9,24,25]. Nutrition support evolved from adjunctive care to its rightful place in the ABCD mnemonic of early priorities of ICU care: Airway, Breathing, Circulation, Diet.
The 2016 joint publication of the SCCM/ASPEN guidelines includes primarily randomized controlled trial (RCT) data, along with some observational trial data, indexed in any major publication database through December 2013. In these guidelines there were 98 recommendations, of which only 5 were a Level 1A; most of the recommendations were categorized as “expert consensus” [12]. The results of several important clinical trials in the United States and Europe that were underway at the time have since been published and compared to the SCCM/ASPEN relevant recommendations [7]. The results have forced nutrition support clinicians to take a step back and re-examine their practice. For many seasoned clinicians who comprised the nutrition support teams of the 1980s and 1990s, it feels like a return to the basics. Until biology-driven personalized medicine is commonplace and genotype data is readily available to guide nutrition therapy for each critically ill patient, standard enteral feeding that begins slow and proceeds carefully over 5 to 7 days towards 80% of goal caloric intake under judicious monitoring of biochemical and metabolic indices may be the “best practice” today, without risk of harm [15,26]. As in all aspects of clinical care, this practice is not without controversy.
ICU Nutrition Support Controversies Today
Enteral vs Parenteral Nutrition
There is universal consensus that EN is the preferred route for nutrition therapy due to the superior physiological response and both nutritional and non-nutritional benefits [24]. Changes in gut permeability tend to occur as illness progresses and consequences include increased bacterial challenge, risk for multiple organ dysfunction syndrome, and systemic infection. It is best to intervene with nutrition early, defined as within the first 48 hours of ICU admission, while the likelihood of success and opportunity to impact the disease process is greater [12]. Early initiation of feeding provides the necessary nutrients to support gut-associated lymphoid tissue (GALT), mucosal-associated lymphoid tissue (MALT), and preserve gut integrity and microbial diversity [27]. The intestine is an effective barrier against bacteria and intraluminal toxins due to the high rate of enterocyte turnover, the mucus secreted by the goblet cells, and the large amount of protective immunological tissue; 80% of the immunoglobulins are synthesized in the GI tract [28]. Fasting states for procedures or delays in feeding longer than 3 days for any reason may contribute to disruption of intestinal integrity through atrophy and derangements in the physical structure and function of the microvilli and crypts [29]. Intestinal dysfunction leads to increased intestinal permeability and the possibility of bacterial translocation. Intestinal ischemia resulting from shock and sepsis may produce hypoxia and reperfusion injuries further affecting intestinal wall permeability [29]. In surgical patients, early enteral feeding has been found to reduce inflammation, oxidative stress, and the catabolic response to anesthesia and surgical-induced stress, help restore intestinal motility, reverse enteric mucosal atrophy, and improve wound healing [26].
We did not have sufficient data to refute the benefits of EN over PN until the paper by Harvey et al (2014), which reported no difference in mortality or infectious complications in ICU patients receiving EN or PN within 36 hours of admission and for up to 5 days [30]. This was the largest published pragmatic RCT, referred to as the CALORIES trial, which analyzed 2388 patients from 33 ICUs and resulted in controversy over what was an unchallenged approach up until this time. It was only a matter of time before other investigators would set out to confirm or negate this finding, which is what Elke and coworkers (2016) did a few years later [31]. They performed an updated systematic review and meta-analysis to evaluate the overall effect of the route of nutrition (EN versus PN) on clinical outcomes in adult critically ill patients. Similar to the Harvey et al report, they found no difference in mortality between the two routes of nutrition. However, unlike the earlier report, patients receiving EN compared to PN had a significant reduction in the number of infectious complications and ICU length of stay. No significant effect was found for hospital length of stay or days requiring mechanical ventilation. The authors suggest that EN delivery of macronutrients below predefined targets may be responsible as PN is more likely to meet or exceed these targets and overwhelm metabolic capacity in the early days of critical illness [31].
The most recent trial prompting even more discussion about early PN versus early EN in mechanically ventilated ICU patients in shock is the Reignier et al (2018) NUTRIREA-2 trial involving 2410 patients from 44 ICUs in France [32]. The investigators hypothesized that outcomes would be better with early exclusive EN compared to early exclusive PN; their hypothesis was not supported by the results, which found no difference in 28-day mortality or ICU-acquired infections. Also unexpected was the higher cumulative incidence of gastrointestinal complications including vomiting, diarrhea, bowel ischemia, and acute colonic obstruction in the EN group. The trial was stopped early after an interim analysis determined that additional enrollment was not likely to significantly change the results of the trial. Given the similarities between the CALORIES trial and this NUTRIREA-2 trial, clinicians now have mounting evidence that equivalent options for nutrition therapy exist and an appropriate selection should be made based on patient-specific indications and treatment goals. In summary, EN remains preferable to PN for the majority of adult critically ill patients due to crucial support of gut integrity, but the optimal dose or rate of delivery to favorably influence clinical outcomes in the first few days following admission remains unknown.
Use of Supplemental Parenteral Nutrition
Both the nutrition support and bench science communities have learned a great deal about PN over the 4 decades it has been in existence, with the most compelling data coming from more recent trials [31–38]. This is because it has taken many years to recover from the days of hyperalimentation or overfeeding ICU patients by providing excessive calories to meet the elevated energy demands and to reverse the hypercatabolism of critical illness. This approach contributed to the complications of hyperglycemia, hyperlipidemia, increased infectious complications, and liver steatosis, all of which gave PN a negative reputation [37]. We now have adjusted the caloric distribution and the actual formulation of PN using the recent FDA-approved lipid emulsion (Soy, Medium-chain triglycerides, Olive oil, and Fish oil; SMOF) and created protocols for administering it based on specific indications, such as loss of GI integrity or demonstrated intolerance. In general, the advances in PN have led to a safer more therapeutic formulation that has its place in critical illness. Manzanares et al [40] reported a trend toward a decrease in ventilation requirement and mortality when a fish oil–containing lipid emulsion was administered to patients who were receiving nutrition support either enterally or parenterally. The meta-analysis combined all soybean oil–sparing lipid emulsions for comparison with soybean oil and was able to show the trend for improved clinical outcomes with soybean oil–sparing lipid emulsions. The main findings of this meta-analysis were that fish oil–containing lipid emulsions may reduce infections and may be associated with a tendency toward fewer mechanical ventilation days, although not mortality, when compared with soybean oil-based strategies or administration of other alternative lipid emulsions in ICU patients [40]. Recent trial results do not change the recommendation for selecting EN first but do suggest lowering the threshold for using PN when EN alone is insufficient to meet nutrition goals. A systematic review reported no benefit of early supplemental PN over late supplemental PN and cautioned that our continued inability to explain greater infectious morbidity and unresolved organ failure limits any justification for early PN [35].
Protein Quantity and Quality
The practice of providing protein in the range of 1.2–2.0 g/kg actual body weight early in critical illness is suggested by expert consensus in the 2016 SCCM/ASPEN guidelines [12]; however, the evidence for efficacy remains controversial. It is argued that the evidence for benefit comes from observational studies, not from prospective RCTs, and that the patients at high risk are often excluded from study protocols. It has also been suggested that in patients with limited vital organ function increased protein delivery may lead to organ compromise. In a recent (2017) paper, Rooyackers et al discussed the post-hoc analyses of data from the EPANIC Trial stating the statistical correlation between protein intake and outcomes indicate that protein was associated with unfavorable outcomes, possibly by inhibiting autophagy [41].
The nutrition support community may have widely varying approaches to feeding critically ill patients but most experts agree that protein may be the most important macronutrient delivered during critical illness. There is consensus that the hypercatabolism associated with stress induces proteolysis and the loss of lean muscle mass, which may affect clinical and functional outcomes beyond the ICU stay. Using multiple assessment modalities, Puthucheary et al (2013) demonstrated a reduction in the rectus femoris muscle of 12.5% over the first week of hospitalization in the ICU and up to 17.7% by day 10. These numbers imply that sufficient protein of at least 1.2 g/kg/day should be provided to minimize these losses, even if the effect on overall outcome remains unknown [42]. Evidence is lacking for whether or not we can prevent the muscle wasting that occurs in critical illness with increased protein dosing. We also need to better identify the possible risks involved with a high-protein intake at the level of the individual patient. A secondary analysis done by Heyland et al (2013) determined that no specific dose or type of macronutrient was found to be associated with improved outcome [43]. It is clear that more large-scale RCTs of protein/amino acid interventions are needed to prove that these nutrition interventions have favorable effects on clinically important outcomes, including long-term physical function.
Polymeric vs Immune-Modulating Nutrients
The Marik and Zaloga (2008) systematic review on immunonutrition in the critically ill convinced most clinicians that while fish-oil based immunonutrition improves the outcome of medical ICU patients, diets supplemented with arginine, with or without glutamine or fish oils, do not demonstrate an advantage over standard enteral products in general ICU, trauma, or burn patients[44]. What followed these trials examining early formulations of immunonutrition was decades of well-intentioned research dedicated to elucidating the mechanism of action for individual immune-modulating nutrients for various populations, including those with acute lung injury/acute respiratory distress syndrome (ARDS) [45–47], sepsis/systemic inflammatory response syndrome [48–50], head and neck cancer [51], upper and lower GI cancer [52–55], and severe acute pancreatitis [56]. Our understanding of immunonutrition and the administration of this formulation in specific disease conditions has grown considerably yet clinicians are still asking exactly what is the role of immunonutrition and who stands to benefit the most from immune-modulating nutrition therapy. The enteral formulations currently available have a proprietary composition and dosage of individual nutrients which yield unpredictable physiologic effects. In addition, the pervasive problem of underfeeding during hospitalization prevents adequate delivery of physiologic doses of nutrients thus contributing to the widely variable research results.
Prevailing expert opinion today is that the majority of critically ill patients will benefit from nutrition support initiated early and delivered consistently; the standard polymeric formula will suffice for the majority of patients with surgical ICU patients potentially deriving benefit from immunonutrition that supports a reduction in infectious complications [57]. In the recent multiple-treatment meta-analysis performed by Mazaki et al (2015) involving 74 studies and 7572 patients, immunonutrition was ranked first for reducing the incidence of 7 complications according to the surface under the cumulative ranking curve; these were as follows: any infection, 0.86; overall complication, 0.88; mortality, 0.81; wound infection, 0.79; intra-abdominal abscess, 0.98; anastomotic leak, 0.79; and sepsis, 0.92. immunonutrition was ranked second for reducing ventilator-associated pneumonia and catheter-associated urinary tract infection (CAUTI), behind immune-modulating PN. The authors stated that immunonutrition was efficacious for reducing the incidence of complications in GI surgery unrelated to the timing of administration [57]. The 2014 publication of results from the MetaPlus Trial [58]challenged the published recommendations for the use of immunonutrition in the medical critically ill population. This trial used high-protein immunonutrition or standard formula for 301 adult critically ill patients in 14 European ICUs with diagnoses such as pneumonia or infections of the urinary tract, bloodstream, central nervous system, eye, ear, nose or throat, and the skin and soft tissue. Even with higher than average target energy intakes of 70% for the high protein immunonutrition group and 80% for the high protein standard group, there were no statistically significant differences in the primary outcome of new infections, or the secondary outcomes of days on mechanical ventilation, Sequential Organ Failure Assessment scores, or ICU and hospital length of stay. However, the 6-month mortality rate of 28% was higher in the medical subgroup [58]. Using these results, as well as existing publications of negative outcomes in medical ICU patients [44,46], the SCCM/ASPEN Guidelines Committee updated its position in 2016 to suggest that immunonutrition formulations or disease-specific formulations should no longer be used routinely in medical ICU patients, including those with acute lung injury/ARDS [12]. The Committee did suggest that these formulations should be reserved for patients with traumatic brain injury and for surgical ICU patients. The benefit for ICU postoperative patients has been linked to the synergistic effect of fish oil and arginine, which must both be present to achieve outcome benefits. A meta-analysis comprised of 35 trials was conducted by Drover et al [58], who reported that administering an arginine and fish oil-containing formula postoperatively reduced infection (RR 0.78; 95% CI, 0.64 to 0.95; P = 0.01) and hospital length of stay (WMD –2.23, 95% CI, –3.80 to –0.65; P = 0.006) but not mortality, when compared to use of a standard formula. Similar results were reported in a second meta-analysis [56], thus providing supportive evidence for the current SCCM/ASPEN recommendation to use an immune-modulating formula (containing both arginine and fish oils) in the SICU for the postoperative patient who requires EN therapy [12].
Safe, Consistent, and Effective Nutrition Support Therapy
Gastric vs Small Bowel Feeding
There is a large group of critically ill patients in whom impaired gastric emptying presents challenges to feeding; 50% of mechanically ventilated patients demonstrate delayed gastric emptying, and 80% of patients with increased intracranial pressure following head injury [60]. In one prospective RCT, Huang et al (2012) showed that severely ill patients (defined by an APACHE II score > 20) fed by the nasoduodenal route experienced significantly shortened hospital LOS, fewer complications, and improved nutrient delivery compared to similar patients fed by the nasogastric route. Less severely ill patients (APACHE II < 20) showed no differences between nasogastric and nasoduodenal feeding for the same outcomes [61]. A recent systematic review [17] pooled data from 14 trials of 1109 participants who received either gastric or small bowel feeding. Moderate quality evidence suggested that post-pyloric feeding was associated with lower rates of pneumonia compared with gastric feeding (RR 0.65, 95% CI, 0.51 to 0.84). Low-quality evidence showed an increase in the percentage of total nutrient delivered to the patient by post-pyloric feeding (mean difference 7.8%, 95% CI, 1.43 to 14.18). Overall, the authors found a 30% lower rate of pneumonia associated with post-pyloric feeding. There is insufficient evidence to show that other clinically important outcomes such as duration of mechanical ventilation, mortality, or LOS were affected by the site of feeding. The American Thoracic Society and ASPEN, as well as the Infectious Diseases Society of America, have published guidelines in support of small bowel feeding in the ICU setting due to its association with reduced incidence of health care–associated infections, specifically ventilator-associated pneumonia [62]. The experts who developed the SCCM/ASPEN and Canadian guidelines stress that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access [12,21]. The reality in ICU clinical practice is that many centers will begin with gastric feeding, barring absolute contraindications, and carefully monitor the patient for signs of intolerance before moving the feeding tube tip into a post-pyloric location. This follows the general recommendation by experts saying in most critically ill patients, it is acceptable to initiate EN in the stomach [12,21]. Protocols that guide management of risk prevention and intolerance typically recommend head of bed elevation, prokinetic agents, and frequent abdominal assessments [63,64].
Once the decision is made to use a post-pyloric feeding tube for nutrition therapy, the next decision is how to safely place the tube, ensure the tip is in an acceptable small bowel location, and minimize delays in feeding. Challenges related to feeding tube insertion may preclude timely advancement to nutrition goals. Placement of feeding tubes into the post-pyloric position is often done at the bedside by trained nursing or medical staff without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without risks. Success rates of this approach vary greatly depending on the patient population and provider expertise. Placement using endoscopic or fluoroscopic guidance is a safe alternative but usually requires coordinating a transport to the radiologic suite, posing safety risks and possible feeding delays for the patient [65]. Bedside use of an electromagnetic placement device (EMPD), such as Cortrak, provides yet another alternative with reports in the literature of 98% success rates for initial placement in less than 20 minutes. In a multicenter prospective study by Powers et al (2011), only one of 194 patients enrolled had data showing a discrepancy between the original EMPD verification and the final radiograph interpretation, demonstrating a 99.5% agreement between the two readings [20]. Median placement time was 12 minutes and no patient experienced an adverse event related to tube insertion using this device. The ability to monitor the location of the feeding tube tip in real time provides a desirable safety feature for the clinician performing bedside insertions. Nurses should consider incorporating the EMPD into the unit feeding protocol, as this would reduce the time to initiation of feedings with early and accurate tube insertion. Ongoing staff education and experience with the procedure are necessary elements to achieve the high rates of success often reported in the literature [66,67]. Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%, with complication rates of 1% to 3% for inadvertent placement of the feeding tube in the airway alone [65]. Radiographic confirmation of tube placement is advised prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.
Gastric Residual Volume Monitoring
A number of factors impede the delivery of EN in the critical care setting; these include gastrointestinal intolerance, under-prescribing to meet daily requirements, frequent interruptions for procedures, and technical issues with tube placement and maintaining patency [68]. Monitoring gastric residual volumes (GRV) contributes to these factors, yet volumes do not correlate well with incidence of pneumonia [69], measures of gastric emptying, or to the incidence of regurgitation and aspiration [70,71]. However, few studies have highlighted the difficulty of obtaining an accurate GRV due to feeding tube tip location, patient position, and type of tube [69]. Several high quality studies have demonstrated that raising the cutoff value for GRV from a lower number of 50–150 mL to a higher number of 250–500 mL does not increase risk for regurgitation, aspiration, or pneumonia [70,71]. A lower cutoff value for GRV does not protect the patient from complications, often leads to inappropriate cessation, and may adversely affect outcome through reduced volume of EN infused [72]. Gastric residual volumes in the range of 200–500 mL should raise concern and lead to the implementation of measures to reduce risk of aspiration, but automatic cessation of feeding should not occur for GRV < 500 mL in the absence of other signs of intolerance [12,69]. Metheny et al (2012) conducted a survey in which more than 97% of nurses responded that they assessed intolerance by measuring GRV; the most frequently cited threshold levels for interrupting feedings were 200 mL and 250 mL [73]. While threshold levels varied widely, only 12.6% of the nurse respondents reported allowing GRV up to 500 mL before interrupting feedings. While monitoring GRV is unnecessary with small bowel feeding, the location of the feeding tube tip should be questioned if gastric contents are obtained from a small bowel tube. The use of GRV as a parameter for trending may also yield important information regarding tolerance of feeding when the patient is unable to communicate abdominal discomfort. Other objective measures to use in the assessment of tolerance include an abdominal exam with documentation of changes in bowel sounds, expanding girth, tenderness or firmness on palpation, increasing nasogastric output, and vomiting [12,68]. If there are indications of intolerance, it is appropriate to divert the tip of the feeding tube into the distal small bowel as discussed previously.
Trophic vs Full Feeding
For the patient with low nutrition risk, there is a lack of convincing data to support an aggressive approach to feeding, either EN or PN, in the first week of critical illness [7]. In recent years, results of several trials suggest early goal-directed feeding in this population may cause net harm with increased morbidity and mortality. When discussing recent controversies in critical care nutrition, one must mention the two schools of thought when it comes to full versus limited energy provision in the first week following ICU admission. Studies in animals and humans have shown a trophic effect of enteral nutrients on the integrity of the gut mucosa, a finding that has provided the rationale for instituting enteral nutrition early during critical illness [15]. However, the inability to provide enteral nutrition early may be a marker of the severity of illness (ie, patients who can be fed enterally are less ill than those who cannot) rather than a mediator of complications and poor outcomes. Compher et al (2017) stated that greater nutritional intake is associated with lower mortality and faster time to discharge alive in high-risk, chronic patients but does not seem to be significant in nutritionally low-risk patients [74]. The findings of the EPaNIC and EDEN trials raised concern that targeting goals that meet full energy needs early in critical illness does not provide benefit and may cause harm in some populations or settings [32,75]. The EDEN trial [32] left us believing that trophic feeding at 10–20 mL/hr may be just as effective as any feeding in the first few days of critical illness striving for 15% to 20% of daily goal calories. After establishing tolerance, advancing daily intake to > 50% to 65% of goal calories, and up to 80% for the highest risk patients, may be required to prevent intestinal permeability and achieve positive clinical outcomes [33].
The systematic review and meta-analysis performed by Al-Dorzi et al (2016) adds further evidence for judicious advancement of EN for critically ill patients [76]. The authors reported finding no association between the dose of caloric intake and hospital mortality. Furthermore, a lower caloric intake resulted in lower risk of bloodstream infections and the need for renal replacement therapy (in 5 of the 21 trials only). As with many other meta-analyses, the authors reported that their results are most assuredly impacted by the heterogeneity in design, feeding route, and dose prescribed and delivered [16,76,77]. Other recent trials such as Arabi et al (2015) that enrolled 894 patients with different feeding targets further confirmed that there is no difference in outcome between groups when it comes to moderate (40% to 60% of goal) vs high (70% to 100% of goal) energy intake, infection rates, or 90-day mortality. The authors summarized their findings saying feeding closer to target is associated with better outcomes compared with severe underfeeding [78]. This adds to the controversy when considering the findings of still other RCTs or meta-analyses that evaluated minimal or trophic feeding versus standard feeding rates [9,46,77]. The meta-analysis performed by Marik and Hooper concluded that there were no differences in the risk of acquired infections, hospital mortality, ICU LOS, or ventilator-free days whether patients received intentional hypocaloric or normocaloric nutrition support [9]. Similarly, there was no significant difference in overall mortality between the underfeeding and full-feeding groups (OR, 0.94; 95% CI, 0.74–1.19; I2 = 26.6%; P = 0.61) in the meta-analysis done by Choi et al (2015), although only 4 trials were included to ensure homogeneity of the population and the intervention [77]. Furthermore, the hospital LOS and ICU LOS did not differ between the 2 groups, nor did any other secondary clinical outcome, leading the authors to conclude that calorie intake of the initial EN support for ICU patients had no bearing on relevant outcomes.
Recent studies have attempted to correlate caloric intake and patient outcomes without success; achieving 100% of caloric goal has not favorably impacted morbidity and mortality. Evidence suggests that intake greater than 65% to 70% of daily caloric requirement in the first 7 to 10 days of ICU stay may be associated with poorer outcomes, particularly when parenteral nutrition is used to supplement intake to achieve the caloric target [33–35].
Conclusion
In this review we described current ICU controversies surrounding nutrition therapy and briefly discussed the data that support more than one course of action. A summary of key points covered is presented in the Table.
Disclaimer: The views expressed in this paper are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government.
Corresponding author: Mary S. McCarthy, PhD, RN, CNSC, 1611 Nisqually St, Steilacoom, WA 98388.
Financial disclosures: None.
1. Hippocratic Oath. Translated by Michael North, National Library of Medicine, 2002.
2. Nightingale F. Notes on Nursing. What it is and what it is not. Radford, VA: Wilder Publications, LLC;2007
3. White JV, Guenter P, Jensen G, et al; the Academy Malnutrition Work Group; the ASPEN Malnutrition Task Force; and the ASPEN Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–83.
4. Hooper MH, Marik PE. Controversies and misconceptions in Intensive Care Unit nutrition. Clin Chest Med 2015;36:409–18.
5. Patel JJ, Codner P. Controversies in critical care nutrition support. Crit Care Clin 2016;32:173–89.
6. Rosenthal MD, Vanzant EL, Martindale RG, Moore FA. Evolving paradigms in the nutritional support of critically ill surgical patients. Curr Probl Surg 2015;52:147–82.
7. McCarthy MS, Warren M, Roberts PR. Recent critical care nutrition trials and the revised guidelines: do they reconcile? Nutr Clin Pract 2016;31:150–4.
8. Barker LA, Gray C, Wilson L, et al. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr 2013;67: 802–807.
9. Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 2016;42:316–323.
10. Patkova A, Joskova V, Havel E, et al. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr 2017;8:624–34.
11. Wong CS, Aly EH. The effects of enteral immunonutrition in upper gastrointestinal surgery: a systematic review and meta-analysis. Int J Surg 2016;29:137–50.
12. McClave SA, Taylor BE, Martindale RG, et al; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society of Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr 2016; 40:159–211.
13. Ammori BJ. Importance of the early increase in intestinal permeability in critically ill patients. Eur J Surg 2002;168:660–1.
14. Vazquez-Sandoval A, Ghamande S, Surani S. Critically ill patients and gut motility: are we addressing it? World J Gastrointest Pharmacol Ther 2017;8:174–9.
15. Patel JJ, Martindale RG, McClave SA. Controversies surrounding critical care nutrition: an appraisal of permissive underfeeding, protein, and outcomes. JPEN J Parenter Enteral Nutr 2017; 148607117721908.
16. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg 2014;219:1078–87.
17. Alkhawaja S, Martin C, Butler RJ, Gwadry-Sridhar F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database of Syst Rev 2015; CD008875.
18. Davies AR, Morrison SS, Bailey MJ, et al ; ENTERIC Study Investigators; ANZICS Clinical Trials Group. A multi-center randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med 2012;40:2342–8.
19. Hsu CW, Sun SF, Lin SL, et al. Duodenal versus gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med 2009;37:1866–72.
20. Powers J, Luebbehusen M, Spitzer T, et al. Verification of an electromagnetic placement device compared with abdominal radiograph to predict accuracy of feeding tube placement. JPEN J Parenter Enteral Nutr 2011;35:535–9.
21. Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract 2014; 29:29–43.22. Kreymann K, Berger M, Deutz N. et al; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006;25:210–23.
23. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Ent Nutr 2009;33:277–316.
24. McClave SA, Martindale RG, Rice TW, Heyland DK. Feeding the critically ill patient. Crit Care Med 2014;42:2600–10.
25. Tian F, Gao X, Wu C, et al. Initial energy supplementation in critically ill patients receiving enteral nutrition: a systematic review and meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr 2017;26:11–9.
26. Martindale RG, Warren M. Should enteral nutrition be started in the first week of critical illness? Curr Opin Clin Nutr Metab Care 2015;18:202–6.
27. McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract 2009;24:305–15.
28. Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr 2007;31:461–82.
29. Seron-Arbeloa C, Zamora-Elson M, Labarta-Monzon L, Mallor-Bonet T. Enteral nutrition in critical care. J Clin Med Res 2013;5:1-11.
30. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673–84.
31. Elke G, van Zanten AR, Lemieux M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117.
32. Reignier J, Boisramé-Helms J, Brisard L, et al. Enteral versus parenteral nutrition in ventilated adults with shock: a randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133–43.
33. Rice TW , Wheeler AP, Thompson BT et al;National Heart, Lung, and Blood Institute, Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307:795–803.
34. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R258.
35. Bost RB, Tjan DH, van Zanten AR. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Ann Intensive Care 2014;4:31.
36. Casaer MP, Mesotten D, Hermans G, et al. Early verus late parenteral nutrition in critically ill adults. N Eng J Med 2011;365:506–17.
37. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Eng J Med 2014;371:1673–84.
38. Manzanares W, Dhaliwal R, Jurewitsch B, et al. Parenteral fish oil lipid emulsions in the critically ill: A systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014;38:20–8.
39. Oshima T, Heidegger CP, Pichard C. Supplemental parenteral nutrition is the key to prevent energy deficits in critically ill patients. Nutr Clin Prac 2016;31:432–7.
40. Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care 2015;19:167.
41. Rooyackers O, Sundström Rehal M, Liebau F, et al. High protein intake without concerns? Crit Care 2017;21:106.
42. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600.
43. Heyland D, Muscedere J, Wischmeyer PE, et al; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97.
44. Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med 2008;34:1980–90.
45. Gadek JE, DeMichele SJ, Karlstad MD, et al; Enteral Nutrition in ARDS Study Group. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med 1999;27:1409–20.
46. Rice TW, Wheeler AP, Thompson BT, et al; NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306:1574–81.
47. Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006;34:1033–38.
48. Atkinson S, Sieffert E, Bihari D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy’s Hospital Intensive Care Group. Crit Care Med 1998;26:1164–72.
49. Galbán C, Montejo JC, Mesejo A, et al. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 2000;28:643–8.
50. Weimann A, Bastian L, Bischoff WE, et al. Influence of arginine, omega-3 fatty acids and nucleotide-supplemented enteral support on systemic inflammatory response syndrome and multiple organ failure in patients after severe trauma. Nutrition 1998;14:165–72.
51. van Bokhorst-De Van Der Schueren MA, Quak JJ, von Blomberg-van der Flier BM, et al. Effect of perioperative nutrition with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am J Clin Nutr 2001;73:323–32.
52. Cerantola Y, Hübner M, Grass F, et al. Immunonutrition in gastrointestinal surgery. Br J Surg 2011;98:37–48.
53. Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr 2010;34:378–86.
54. Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid–supplemented enteral nutrition vs. standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg 2012;99:346–55.
55. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg 2006;30:1592–604.
56. Pearce CB, Sadek SA, Walters AM, et al. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP 2006;7:361–71.
57. Mazaki T, Ishii Y, Murai I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: a multiple treatments meta-analysis. Ann Surg 2015;261:662–9.
58. van Zanten ARH, Sztark F, Kaisers UX, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high protein enteral nutrition and nosocomial infections in the ICU. JAMA 2014;312:514–24.
59. Drover JW, Dhaliwal R, Weitzel L, et al. Perioperative use of arginine supplemented diets: a systematic review of the evidence. J Am Coll Surg 2011;212:385–99.
60. Stupak D, Abdelsayed GG, Soloway GN. Motility disorders of the upper gastrointestinal tract in the intensive care unit: pathophysiology and contemporary management. J Clin Gastroenterol 2012;46:449–56.
61. Huang HH, Chang SJ, Hsu CW, et al. Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet 2012;112:1138–46.
62. American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416.
63. Heyland DK, Cahill NE, Dhaliwal R, et al. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr 2010;34:675–84.
64. Landzinski J, Kiser TH, Fish DN, et al. Gastric motility function in critically ill patients tolerant vs intolerant to gastric nutrition. JPEN J Parenter Enteral Nutr 2008;32:45–50.
65. de Aguilar-Nascimento JE, Kudsk KA. Use of small bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care 2007;10:291–6.
66. Boyer N, McCarthy MS, Mount CA. Analysis of an electromagnetic tube placement device vs a self-advancing nasal jejunal device for postpyloric feeding tube placement . J Hosp Med 2014;9:23–8.
67. Metheny NA, Meert KL. Effectiveness of an electromagnetic feeding tube placement device in detecting inadvertent respiratory placement. Am J Crit Care 2014;23:240–8.
68. Montejo JC, Miñambres E, Bordejé L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 2010;36:1386–93.
69. Hurt RT, McClave SA. Gastric residual volumes in critical illness: what do they really mean? Crit Care Clin 2010;26:481–90.
70. Poulard F, Dimet J, Martin-Lefevre L, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr 2010;34:125–30.
71. Reignier J, Mercier E, Gouge AL, et al; Clinical Research in Intensive Care and Sepsis (CRICS) Group. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013;309:249–56.
72. Williams TA, Leslie GD, Leen T, et al. Reducing interruptions to continuous enteral nutrition in the intensive care unit: a comparative study. J Clin Nurs 2013;22:2838-2848.
73. Metheny NA, Stewart BJ, Mills AC. Blind insertion of feeding tubes in intensive care units: a national survey. Am J Crit Care 2012;21:352–360.
74. Compher C, Chittams J, Sammarco T, et al. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: A multicenter, multinational observational study. Crit Care Med 2017;45:156–163.
75. Casaer MP, Wilmer A, Hermans G, et al. Role of disease and macronutrient dose in the randomized controlled EPANIC Trial: a post hoc analysis. Am J Resp Crit Care Med 2013;187:247–55.
76. Al-Dorzi HM, Albarrak A, Ferwana M, et al. Lower versus higher dose of enteral caloric intake in adult critically ill patients: a systematic review and meta-analysis. Crit Care 2016;20:358.
77. Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2015;39:291–300.
78. Arabi YM, Aldawood AS, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med 2015;372:2398–408.
1. Hippocratic Oath. Translated by Michael North, National Library of Medicine, 2002.
2. Nightingale F. Notes on Nursing. What it is and what it is not. Radford, VA: Wilder Publications, LLC;2007
3. White JV, Guenter P, Jensen G, et al; the Academy Malnutrition Work Group; the ASPEN Malnutrition Task Force; and the ASPEN Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–83.
4. Hooper MH, Marik PE. Controversies and misconceptions in Intensive Care Unit nutrition. Clin Chest Med 2015;36:409–18.
5. Patel JJ, Codner P. Controversies in critical care nutrition support. Crit Care Clin 2016;32:173–89.
6. Rosenthal MD, Vanzant EL, Martindale RG, Moore FA. Evolving paradigms in the nutritional support of critically ill surgical patients. Curr Probl Surg 2015;52:147–82.
7. McCarthy MS, Warren M, Roberts PR. Recent critical care nutrition trials and the revised guidelines: do they reconcile? Nutr Clin Pract 2016;31:150–4.
8. Barker LA, Gray C, Wilson L, et al. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr 2013;67: 802–807.
9. Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 2016;42:316–323.
10. Patkova A, Joskova V, Havel E, et al. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr 2017;8:624–34.
11. Wong CS, Aly EH. The effects of enteral immunonutrition in upper gastrointestinal surgery: a systematic review and meta-analysis. Int J Surg 2016;29:137–50.
12. McClave SA, Taylor BE, Martindale RG, et al; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society of Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr 2016; 40:159–211.
13. Ammori BJ. Importance of the early increase in intestinal permeability in critically ill patients. Eur J Surg 2002;168:660–1.
14. Vazquez-Sandoval A, Ghamande S, Surani S. Critically ill patients and gut motility: are we addressing it? World J Gastrointest Pharmacol Ther 2017;8:174–9.
15. Patel JJ, Martindale RG, McClave SA. Controversies surrounding critical care nutrition: an appraisal of permissive underfeeding, protein, and outcomes. JPEN J Parenter Enteral Nutr 2017; 148607117721908.
16. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg 2014;219:1078–87.
17. Alkhawaja S, Martin C, Butler RJ, Gwadry-Sridhar F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database of Syst Rev 2015; CD008875.
18. Davies AR, Morrison SS, Bailey MJ, et al ; ENTERIC Study Investigators; ANZICS Clinical Trials Group. A multi-center randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med 2012;40:2342–8.
19. Hsu CW, Sun SF, Lin SL, et al. Duodenal versus gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med 2009;37:1866–72.
20. Powers J, Luebbehusen M, Spitzer T, et al. Verification of an electromagnetic placement device compared with abdominal radiograph to predict accuracy of feeding tube placement. JPEN J Parenter Enteral Nutr 2011;35:535–9.
21. Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract 2014; 29:29–43.22. Kreymann K, Berger M, Deutz N. et al; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006;25:210–23.
23. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Ent Nutr 2009;33:277–316.
24. McClave SA, Martindale RG, Rice TW, Heyland DK. Feeding the critically ill patient. Crit Care Med 2014;42:2600–10.
25. Tian F, Gao X, Wu C, et al. Initial energy supplementation in critically ill patients receiving enteral nutrition: a systematic review and meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr 2017;26:11–9.
26. Martindale RG, Warren M. Should enteral nutrition be started in the first week of critical illness? Curr Opin Clin Nutr Metab Care 2015;18:202–6.
27. McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract 2009;24:305–15.
28. Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr 2007;31:461–82.
29. Seron-Arbeloa C, Zamora-Elson M, Labarta-Monzon L, Mallor-Bonet T. Enteral nutrition in critical care. J Clin Med Res 2013;5:1-11.
30. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673–84.
31. Elke G, van Zanten AR, Lemieux M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117.
32. Reignier J, Boisramé-Helms J, Brisard L, et al. Enteral versus parenteral nutrition in ventilated adults with shock: a randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133–43.
33. Rice TW , Wheeler AP, Thompson BT et al;National Heart, Lung, and Blood Institute, Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307:795–803.
34. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R258.
35. Bost RB, Tjan DH, van Zanten AR. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Ann Intensive Care 2014;4:31.
36. Casaer MP, Mesotten D, Hermans G, et al. Early verus late parenteral nutrition in critically ill adults. N Eng J Med 2011;365:506–17.
37. Harvey SE, Parrott F, Harrison DA, et al; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Eng J Med 2014;371:1673–84.
38. Manzanares W, Dhaliwal R, Jurewitsch B, et al. Parenteral fish oil lipid emulsions in the critically ill: A systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014;38:20–8.
39. Oshima T, Heidegger CP, Pichard C. Supplemental parenteral nutrition is the key to prevent energy deficits in critically ill patients. Nutr Clin Prac 2016;31:432–7.
40. Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care 2015;19:167.
41. Rooyackers O, Sundström Rehal M, Liebau F, et al. High protein intake without concerns? Crit Care 2017;21:106.
42. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600.
43. Heyland D, Muscedere J, Wischmeyer PE, et al; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97.
44. Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med 2008;34:1980–90.
45. Gadek JE, DeMichele SJ, Karlstad MD, et al; Enteral Nutrition in ARDS Study Group. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med 1999;27:1409–20.
46. Rice TW, Wheeler AP, Thompson BT, et al; NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306:1574–81.
47. Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006;34:1033–38.
48. Atkinson S, Sieffert E, Bihari D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy’s Hospital Intensive Care Group. Crit Care Med 1998;26:1164–72.
49. Galbán C, Montejo JC, Mesejo A, et al. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 2000;28:643–8.
50. Weimann A, Bastian L, Bischoff WE, et al. Influence of arginine, omega-3 fatty acids and nucleotide-supplemented enteral support on systemic inflammatory response syndrome and multiple organ failure in patients after severe trauma. Nutrition 1998;14:165–72.
51. van Bokhorst-De Van Der Schueren MA, Quak JJ, von Blomberg-van der Flier BM, et al. Effect of perioperative nutrition with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am J Clin Nutr 2001;73:323–32.
52. Cerantola Y, Hübner M, Grass F, et al. Immunonutrition in gastrointestinal surgery. Br J Surg 2011;98:37–48.
53. Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr 2010;34:378–86.
54. Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid–supplemented enteral nutrition vs. standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg 2012;99:346–55.
55. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg 2006;30:1592–604.
56. Pearce CB, Sadek SA, Walters AM, et al. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP 2006;7:361–71.
57. Mazaki T, Ishii Y, Murai I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: a multiple treatments meta-analysis. Ann Surg 2015;261:662–9.
58. van Zanten ARH, Sztark F, Kaisers UX, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high protein enteral nutrition and nosocomial infections in the ICU. JAMA 2014;312:514–24.
59. Drover JW, Dhaliwal R, Weitzel L, et al. Perioperative use of arginine supplemented diets: a systematic review of the evidence. J Am Coll Surg 2011;212:385–99.
60. Stupak D, Abdelsayed GG, Soloway GN. Motility disorders of the upper gastrointestinal tract in the intensive care unit: pathophysiology and contemporary management. J Clin Gastroenterol 2012;46:449–56.
61. Huang HH, Chang SJ, Hsu CW, et al. Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet 2012;112:1138–46.
62. American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416.
63. Heyland DK, Cahill NE, Dhaliwal R, et al. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr 2010;34:675–84.
64. Landzinski J, Kiser TH, Fish DN, et al. Gastric motility function in critically ill patients tolerant vs intolerant to gastric nutrition. JPEN J Parenter Enteral Nutr 2008;32:45–50.
65. de Aguilar-Nascimento JE, Kudsk KA. Use of small bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care 2007;10:291–6.
66. Boyer N, McCarthy MS, Mount CA. Analysis of an electromagnetic tube placement device vs a self-advancing nasal jejunal device for postpyloric feeding tube placement . J Hosp Med 2014;9:23–8.
67. Metheny NA, Meert KL. Effectiveness of an electromagnetic feeding tube placement device in detecting inadvertent respiratory placement. Am J Crit Care 2014;23:240–8.
68. Montejo JC, Miñambres E, Bordejé L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 2010;36:1386–93.
69. Hurt RT, McClave SA. Gastric residual volumes in critical illness: what do they really mean? Crit Care Clin 2010;26:481–90.
70. Poulard F, Dimet J, Martin-Lefevre L, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr 2010;34:125–30.
71. Reignier J, Mercier E, Gouge AL, et al; Clinical Research in Intensive Care and Sepsis (CRICS) Group. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013;309:249–56.
72. Williams TA, Leslie GD, Leen T, et al. Reducing interruptions to continuous enteral nutrition in the intensive care unit: a comparative study. J Clin Nurs 2013;22:2838-2848.
73. Metheny NA, Stewart BJ, Mills AC. Blind insertion of feeding tubes in intensive care units: a national survey. Am J Crit Care 2012;21:352–360.
74. Compher C, Chittams J, Sammarco T, et al. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: A multicenter, multinational observational study. Crit Care Med 2017;45:156–163.
75. Casaer MP, Wilmer A, Hermans G, et al. Role of disease and macronutrient dose in the randomized controlled EPANIC Trial: a post hoc analysis. Am J Resp Crit Care Med 2013;187:247–55.
76. Al-Dorzi HM, Albarrak A, Ferwana M, et al. Lower versus higher dose of enteral caloric intake in adult critically ill patients: a systematic review and meta-analysis. Crit Care 2016;20:358.
77. Choi EY, Park DA, Park J. Calorie intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2015;39:291–300.
78. Arabi YM, Aldawood AS, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med 2015;372:2398–408.