User login
Cirrhosis and its complications are among the top 10 causes of death in the United States.1,2 One of the most common complications of cirrhosis is ascites, an abnormal accumulation of fluid in the peritoneal cavity.3 Although ascites can be of nonhepatic origin, in approximately 85% of cases, the cause is cirrhosis.2,4
Developing in some 60% of cirrhosis patients within 10 years,3 ascites indicates disease progression from compensated to decompensated cirrhosis.5 Mortality from ascites is approximately 15% in the first year and 44% by the fifth year, so referral for liver transplant evaluation is often indicated.2
Frequently, however, patients do not meet the criteria for transplantation because of comorbidities such as morbid obesity, severe cardiac or pulmonary disease, severe malignancy, chemical dependency, or lack of caregiver support.6 Primary care clinicians need to know about the management of ascites in chronic liver disease in order to meet the significant ongoing health care needs of these patients.
This article reviews the diagnosis and treatment of cirrhosis-related ascites and discusses one particularly life-threatening infection—spontaneous bacterial peritonitis (SBP)—to which these patients are susceptible.
CASE A 52-year-old African-American man presented to the emergency department (ED) with complaints of severe diffuse abdominal pain, worsening over the past few days, as well as nausea and vomiting. History was significant for hepatitis C–related cirrhosis, unresponsive to antiviral treatment, and liver disease complications that included hepatic encephalopathy; portal hypertension; ascites requiring large-volume paracentesis every one to two weeks (most recently, six days earlier); esophageal varices (status postbanding by esophagogastroduodenoscopy); portal hypertensive gastropathy; and gastric varices.
Due to decompensated cirrhosis, the patient had previously undergone extensive screening, radiologic imaging, and laboratory testing, and was found by a multidisciplinary selection committee to be an acceptable liver transplant candidate. He was actively listed for transplantation.
Other significant history included hypertension; sleep apnea; gastroesophageal reflux; osteopenia; zinc, vitamin A, and vitamin D deficiencies; thrombocytopenia; and anemia of chronic disease (liver disease–related). Surgical history was negative. The patient reported no known drug allergies and was taking spironolactone (50 mg/d) and furosemide (20 mg/d).
Physical exam was notable for a low-grade fever of 99.1ºF; blood pressure, 132/81 mm Hg; heart rate, 84 beats/min; icteric sclerae; and moderate distress related to the patient’s abdominal pain, which worsened with deep palpation. The abdomen was distended, with ascites present as indicated by a positive fluid wave test. Bowel sounds were hypoactive. The patient was alert and oriented without asterixis; mental state was within normal limits.
Continue for pathophysiology of ascites >>
PATHOPHYSIOLOGY OF ASCITES
In a patient with cirrhosis, blood flow is reduced through the scarred liver and becomes retrograde to the normal flow pattern, causing portal hypertension. Portal hypertension causes vasodilators, such as nitric oxide, to be produced, leading to vasodilation of the splanchnic arterial system. Eventually, as vasodilation increases, the arterial receptors sense a decreased amount of blood flow in this part of the circulation. The body activates various systems (sympathetic nervous, antidiuretic hormone, and renin-angiotensin-aldosterone), resulting in increased water and sodium absorption and renal vasoconstriction. In turn, intestinal permeability and pressure in the capillaries respond, allowing fluid—ascites—to move into the peritoneal cavity.4,5,7
SPONTANEOUS BACTERIAL PERITONITIS
Developing in approximately 25% of patients with cirrhosis and ascites,8 spontaneous bacterial peritonitis (SBP) is thought to occur by translocation of intestinal bacteria moving through the mesenteric lymph nodes into the bloodstream and other body fluids (eg, ascites).4,7,9Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumonia are the bacteria most often responsible for SBP.2
If left untreated or treated too late, SBP can eventually lead to sepsis and septic shock.4,7,9 About 30% of cirrhosis patients with SBP will die of it or related complications;8 the one-year survival rate is 30% to 50% and the two-year survival rate, 25% to 30%.9 Mortality is increased by up to 50% in hospitalized patients with SBP.3,10,11
DIAGNOSIS
Ascites
In patients with cirrhosis, typical signs and symptoms of ascites include weight gain, increased abdominal girth and fullness, dullness to abdominal percussion, peripheral edema, and a positive fluid wave test.2,4
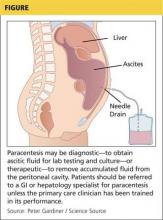
In both inpatient and outpatient settings, ascites should be sampled by diagnostic abdominal paracentesis (see Figure), which requires 30 to 50 mL of fluid.12 Laboratory analysis should include white blood cell count with differential, serum-ascites albumin gradient (SAAG), and total protein. If infection is suspected, samples should be sent for culture, using blood culture bottles, as well as for gram staining.2,12
SAAG is calculated by measuring the albumin concentration in ascitic fluid and serum specimens taken on the same day and then subtracting the ascitic fluid value from the serum value. With 97% accuracy, a SAAG ≥ 1.1 g/dL indicates portal hypertension, meaning that the ascites is likely hepatic in origin.2,12
Continue for SBP >>
SBP
Approximately 87% of patients with SBP will have signs or symptoms of infection,3 but symptoms can be very vague, so careful attention to detail and thorough assessment are necessary (see Table 1).2,4,7,12 An elevated absolute polymorphonuclear (PMN) leukocyte cell count of ≥ 250 cells/µL and a positive ascitic culture, with no other apparent source of intra-abdominal infection, are diagnostic for SBP.2
Any cirrhotic patient with ascites who presents with two or more of the following criteria for systemic inflammatory response syndrome should be evaluated for an infectious cause:13
• Temperature > 100.4°F or < 96.8°F
• Heart rate > 90 beats/min
• Tachypnea (respiratory rate > 20 breaths/min) or hyperventilation (arterial carbon dioxide tension [PaCO2] < 32 mm Hg)
• Abnormal white blood cell count (> 12,000/µL or < 4,000/µL or > 10% immature neutrophils [band forms])
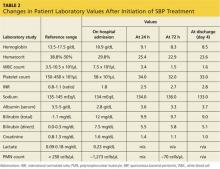
CASE Abdominal x-ray, CT, and laboratory testing were ordered in the ED (see Table 2 for laboratory test results). Imaging revealed multiple loops of small bowel in the midabdomen, with several air-fluid levels and a paucity of gas in the rectum. These findings were consistent with small bowel obstruction. In addition, laboratory data were significant for leukocytosis (higher than the patient’s usual level), worsening hyperbilirubinemia and hypoalbuminemia, elevated serum creatinine level indicating acute kidney injury (AKI), and lactic acidosis. The patient was admitted to the hospital for further management.
Because of his severe abdominal pain and leukocytosis, bedside paracentesis was performed. He was found to have an absolute PMN cell count of approximately 1,273 cells/µL, suggesting a diagnosis of SBP. The patient’s status on the transplant list was changed to inactive due to the infection.
Next page: Treatment >>
TREATMENT
Ascites
First-line treatment for patients with ascites includes dietary sodium restriction (maximum 2,000 mg/d) and oral diuretics (spironolactone with or without furosemide).2,5 This regimen is effective for 90% of patients.5 Typically, a ratio of 100 mg of spironolactone to 40 mg of furosemide is ideal to promote adequate diuresis while maintaining normal electrolyte balance. Doses can be increased every three to five days, with maximum doses of 400 mg/d of spironolactone and 160 mg/d of furosemide.2 Renal function and sodium levels should be monitored closely and diuretic dosing adjusted based on clinical presentation to avoid volume depletion, which puts patients at risk for AKI and hyponatremia.5,12 Diuretics should not be given in cases of AKI (creatinine level > 2 mg/dL), kidney failure (ie, patients on dialysis), acute infection, uncontrolled encephalopathy, or severe hyponatremia (sodium < 120 mEq/L).2,4
Refractory ascites
Approximately 10% of patients develop refractory ascites when the condition becomes unresponsive to diuretics or when adverse effects preclude the use of diuretics.5 In such cases, large-volume paracentesis (> 5 L removed) is the standard treatment. The patient should be monitored closely during the procedure; blood pressure can decrease drastically due to the large fluid loss. Vital signs should be checked every 15 min to 30 min for the first hour postprocedure, and then hourly if signs remain stable. Albumin should be administered (6-8 g/L of fluid removed) to increase circulating fluid volume.2 The patient’s renal function (ie, serum creatinine level and urinary output) should also be monitored closely, as dehydration and/or AKI can occur.4 Complications of paracentesis are uncommon but may include bleeding, infection, and bowel perforation. No data support the routine administration of blood products before paracentesis.2
Treatment of SBP
Broad-spectrum antibiotics, particularly cefotaxime, are effective against approximately 94% of the bacterial flora associated with SBP. Initial treatment with cefotaxime (or ceftriaxone, if cefotaxime is unavailable) at a dose of 2 g IV q8h for seven days is recommended, unless or until culture results identify susceptibility to a specific narrow-spectrum antibiotic.2,4,7,9 After completion of a total of seven days of IV antibiotics, it is cost effective and safe to transition clinically improved patients to an oral antibiotic for SBP prophylaxis (see discussion below).9
A repeat paracentesis performed 48 hours after treatment is initiated is useful in assessing response to treatment, defined as a ≥ 25% decrease in ascitic fluid PMN count.7
Even if the infection is successfully treated, one-third of patients who have an episode of SBP develop renal failure; those who experience a recurrence of SBP within one year have a 50% to 60% mortality rate.4,7,9,14 This is because infection causes vasodilation, with a concomitant decrease in blood volume and compensatory activation of renal vasoconstrictors, so renal function worsens.
A randomized controlled trial found that patients treated with IV cefotaxime with albumin in doses of 1.5 g/kg on day 1 and 1 g/kg on day 3 had significantly lower rates of AKI and mortality than patients who received cefotaxime alone.15 An albumin infusion, given in conjunction with antibiotics, improves blood volume, enhances renal perfusion, and has been shown to decrease mortality from SBP from 29% to 10%.2,12,16
CASE For small bowel obstruction, the patient was made NPO and a nasogastric tube was placed for 36 hours. During this time, he had a bowel movement, diet was advanced, and the obstruction resolved.
At the same time, the patient was started on IV cefotaxime 2 g q8h for treatment of SBP, along with IV albumin and fluids. Diuretics were withheld because of impaired kidney function.
Ascitic fluid was cultured, and Streptococcus viridans was identified. After consultation with the infectious disease service, broader-spectrum vancomycin was added to the patient’s IV antibiotic regimen 24 hours after admission while culture results finalized. Cultures were analyzed after three days and found to be susceptible to ceftriaxone, which was administered 2 g IV q24h for three days, for a total of seven days of IV antibiotics.
The patient’s AKI improved after treatment with IV antibiotics, fluids, and albumin. Seventy-two hours after admission, repeat paracentesis revealed a significant decrease in white blood cells; cultures were negative for infection.
Neutrocytic ascites
In some cases, the bacteria count in ascitic fluid is so low that cultures may be negative.4 This is termed neutrocytic ascites, characterized by an elevated PMN cell count with negative cultures. Patients with neutrocytic ascites should be treated as though they have SBP.2,7
Prophylactic antibiotics
Of patients successfully treated for SBP, 69% will experience another episode within one year; many of these cases can be prevented with the use of prophylactic antibiotics. Historically, quinolones were used for SBP prophylaxis, and they are cost-effective. However, multiorganism drug resistance hasoccurred after quinolone prophylaxis, especially if given long-term.2,4,7
Alternatives to quinolones include trimethoprim/sulfamethoxazole double strength or ciprofloxacin 500 mg/d.2 Renal dose adjustment is necessary for patients with acute or chronic kidney disease and can usually be guided by a pharmacist. One study found norfloxacin and trimethoprim-sulfamethoxazole to be similarly effective for SBP prevention, but trimethoprim-sulfamethoxazole was noted to be more cost-effective.10
In patients with active gastrointestinal bleeding, IV ceftriaxone 1 g/d for seven days can be given for SBP prevention. In patients with low ascitic protein and/or history of prior SBP, norfloxacin 400 mg/d is an option.
Continue for conclusion >>
CASE On day 4 of hospitalization, the patient was determined to be stable for discharge, with close follow-up. After completion of three days of IV antibiotics on an outpatient basis, the patient began ciprofloxacin (500 mg/d) for SBP prophylaxis. His status on the transplant list was reactivated and, two weeks later, the patient received a liver transplant.
CONCLUSION
As the number of patients living with cirrhosis increases, it is very important for health care providers to appropriately identify and treat this patient population. Ascites and SBP can be deadly but can also be treated effectively. Ideal management includes early identification, proper laboratory testing and radiologic imaging, treatment with fluids and albumin, antibiotic administration in the acute care setting, and antibiotics for SBP prophylaxis. Diuretics should be used with caution and doses adjusted based on clinical judgment and the patient’s presentation. Patients should be referred for liver transplant evaluation. Early identification and treatment of ascites and SBP are essential for patient survival.
REFERENCES
1. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375-382.
2. Runyon BA. American Association for the Study of Liver Diseases practice guideline. Management of adult patients with ascites due to cirrhosis: update 2012. www.aasld.org/sites/default/files/guideline_documents/adultascitesenhanced.pdf. Accessed March 24, 2015.
3. European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397-417.
4. Sargent S. The management and nursing care of cirrhotic ascites. Br J Nurs. 2006;15(4):212-219.
5. Sargent S. Management of patients with advanced liver cirrhosis. Nurs Stand. 2006;21(11):48-56.
6. Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. American Association for the Study of Liver Diseases and the American Society of Transplantation. Evaluation for liver transplantation in adults: 2013 practice guideline. Hepatology. 2014;59(3):1144-65.
7. Loo NM, Souza FF, Garcia-Tsao G. Non-hemorrhagic acute complications associated with cirrhosis and portal hypertension. Best Pract Res Clin Gastroenterol. 2013;27(5):665-678.
8. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.
9. Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):
77-93.
10. Lontos S, Gow PJ, Vaughan RB, Angus PW. Norfloxacin and trimethoprim-sulfamethoxazole therapy have similar efficacy in prevention of spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2008;23(2): 252-255.
11. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36.
12. Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World J Hepatol. 2013;5(5):251-263.
13. Bone RC, Balk RA, Cerra FB, et al; ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655.
14. Wong F, O’Leary JG, Reddy KR, et al; North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6):1280-1288.
15. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409.
16. Rozga J, Piatek T, Malkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205-217.
Cirrhosis and its complications are among the top 10 causes of death in the United States.1,2 One of the most common complications of cirrhosis is ascites, an abnormal accumulation of fluid in the peritoneal cavity.3 Although ascites can be of nonhepatic origin, in approximately 85% of cases, the cause is cirrhosis.2,4
Developing in some 60% of cirrhosis patients within 10 years,3 ascites indicates disease progression from compensated to decompensated cirrhosis.5 Mortality from ascites is approximately 15% in the first year and 44% by the fifth year, so referral for liver transplant evaluation is often indicated.2
Frequently, however, patients do not meet the criteria for transplantation because of comorbidities such as morbid obesity, severe cardiac or pulmonary disease, severe malignancy, chemical dependency, or lack of caregiver support.6 Primary care clinicians need to know about the management of ascites in chronic liver disease in order to meet the significant ongoing health care needs of these patients.
This article reviews the diagnosis and treatment of cirrhosis-related ascites and discusses one particularly life-threatening infection—spontaneous bacterial peritonitis (SBP)—to which these patients are susceptible.
CASE A 52-year-old African-American man presented to the emergency department (ED) with complaints of severe diffuse abdominal pain, worsening over the past few days, as well as nausea and vomiting. History was significant for hepatitis C–related cirrhosis, unresponsive to antiviral treatment, and liver disease complications that included hepatic encephalopathy; portal hypertension; ascites requiring large-volume paracentesis every one to two weeks (most recently, six days earlier); esophageal varices (status postbanding by esophagogastroduodenoscopy); portal hypertensive gastropathy; and gastric varices.
Due to decompensated cirrhosis, the patient had previously undergone extensive screening, radiologic imaging, and laboratory testing, and was found by a multidisciplinary selection committee to be an acceptable liver transplant candidate. He was actively listed for transplantation.
Other significant history included hypertension; sleep apnea; gastroesophageal reflux; osteopenia; zinc, vitamin A, and vitamin D deficiencies; thrombocytopenia; and anemia of chronic disease (liver disease–related). Surgical history was negative. The patient reported no known drug allergies and was taking spironolactone (50 mg/d) and furosemide (20 mg/d).
Physical exam was notable for a low-grade fever of 99.1ºF; blood pressure, 132/81 mm Hg; heart rate, 84 beats/min; icteric sclerae; and moderate distress related to the patient’s abdominal pain, which worsened with deep palpation. The abdomen was distended, with ascites present as indicated by a positive fluid wave test. Bowel sounds were hypoactive. The patient was alert and oriented without asterixis; mental state was within normal limits.
Continue for pathophysiology of ascites >>
PATHOPHYSIOLOGY OF ASCITES
In a patient with cirrhosis, blood flow is reduced through the scarred liver and becomes retrograde to the normal flow pattern, causing portal hypertension. Portal hypertension causes vasodilators, such as nitric oxide, to be produced, leading to vasodilation of the splanchnic arterial system. Eventually, as vasodilation increases, the arterial receptors sense a decreased amount of blood flow in this part of the circulation. The body activates various systems (sympathetic nervous, antidiuretic hormone, and renin-angiotensin-aldosterone), resulting in increased water and sodium absorption and renal vasoconstriction. In turn, intestinal permeability and pressure in the capillaries respond, allowing fluid—ascites—to move into the peritoneal cavity.4,5,7
SPONTANEOUS BACTERIAL PERITONITIS
Developing in approximately 25% of patients with cirrhosis and ascites,8 spontaneous bacterial peritonitis (SBP) is thought to occur by translocation of intestinal bacteria moving through the mesenteric lymph nodes into the bloodstream and other body fluids (eg, ascites).4,7,9Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumonia are the bacteria most often responsible for SBP.2
If left untreated or treated too late, SBP can eventually lead to sepsis and septic shock.4,7,9 About 30% of cirrhosis patients with SBP will die of it or related complications;8 the one-year survival rate is 30% to 50% and the two-year survival rate, 25% to 30%.9 Mortality is increased by up to 50% in hospitalized patients with SBP.3,10,11
DIAGNOSIS
Ascites
In patients with cirrhosis, typical signs and symptoms of ascites include weight gain, increased abdominal girth and fullness, dullness to abdominal percussion, peripheral edema, and a positive fluid wave test.2,4
In both inpatient and outpatient settings, ascites should be sampled by diagnostic abdominal paracentesis (see Figure), which requires 30 to 50 mL of fluid.12 Laboratory analysis should include white blood cell count with differential, serum-ascites albumin gradient (SAAG), and total protein. If infection is suspected, samples should be sent for culture, using blood culture bottles, as well as for gram staining.2,12
SAAG is calculated by measuring the albumin concentration in ascitic fluid and serum specimens taken on the same day and then subtracting the ascitic fluid value from the serum value. With 97% accuracy, a SAAG ≥ 1.1 g/dL indicates portal hypertension, meaning that the ascites is likely hepatic in origin.2,12
Continue for SBP >>
SBP
Approximately 87% of patients with SBP will have signs or symptoms of infection,3 but symptoms can be very vague, so careful attention to detail and thorough assessment are necessary (see Table 1).2,4,7,12 An elevated absolute polymorphonuclear (PMN) leukocyte cell count of ≥ 250 cells/µL and a positive ascitic culture, with no other apparent source of intra-abdominal infection, are diagnostic for SBP.2
Any cirrhotic patient with ascites who presents with two or more of the following criteria for systemic inflammatory response syndrome should be evaluated for an infectious cause:13
• Temperature > 100.4°F or < 96.8°F
• Heart rate > 90 beats/min
• Tachypnea (respiratory rate > 20 breaths/min) or hyperventilation (arterial carbon dioxide tension [PaCO2] < 32 mm Hg)
• Abnormal white blood cell count (> 12,000/µL or < 4,000/µL or > 10% immature neutrophils [band forms])
CASE Abdominal x-ray, CT, and laboratory testing were ordered in the ED (see Table 2 for laboratory test results). Imaging revealed multiple loops of small bowel in the midabdomen, with several air-fluid levels and a paucity of gas in the rectum. These findings were consistent with small bowel obstruction. In addition, laboratory data were significant for leukocytosis (higher than the patient’s usual level), worsening hyperbilirubinemia and hypoalbuminemia, elevated serum creatinine level indicating acute kidney injury (AKI), and lactic acidosis. The patient was admitted to the hospital for further management.
Because of his severe abdominal pain and leukocytosis, bedside paracentesis was performed. He was found to have an absolute PMN cell count of approximately 1,273 cells/µL, suggesting a diagnosis of SBP. The patient’s status on the transplant list was changed to inactive due to the infection.
Next page: Treatment >>
TREATMENT
Ascites
First-line treatment for patients with ascites includes dietary sodium restriction (maximum 2,000 mg/d) and oral diuretics (spironolactone with or without furosemide).2,5 This regimen is effective for 90% of patients.5 Typically, a ratio of 100 mg of spironolactone to 40 mg of furosemide is ideal to promote adequate diuresis while maintaining normal electrolyte balance. Doses can be increased every three to five days, with maximum doses of 400 mg/d of spironolactone and 160 mg/d of furosemide.2 Renal function and sodium levels should be monitored closely and diuretic dosing adjusted based on clinical presentation to avoid volume depletion, which puts patients at risk for AKI and hyponatremia.5,12 Diuretics should not be given in cases of AKI (creatinine level > 2 mg/dL), kidney failure (ie, patients on dialysis), acute infection, uncontrolled encephalopathy, or severe hyponatremia (sodium < 120 mEq/L).2,4
Refractory ascites
Approximately 10% of patients develop refractory ascites when the condition becomes unresponsive to diuretics or when adverse effects preclude the use of diuretics.5 In such cases, large-volume paracentesis (> 5 L removed) is the standard treatment. The patient should be monitored closely during the procedure; blood pressure can decrease drastically due to the large fluid loss. Vital signs should be checked every 15 min to 30 min for the first hour postprocedure, and then hourly if signs remain stable. Albumin should be administered (6-8 g/L of fluid removed) to increase circulating fluid volume.2 The patient’s renal function (ie, serum creatinine level and urinary output) should also be monitored closely, as dehydration and/or AKI can occur.4 Complications of paracentesis are uncommon but may include bleeding, infection, and bowel perforation. No data support the routine administration of blood products before paracentesis.2
Treatment of SBP
Broad-spectrum antibiotics, particularly cefotaxime, are effective against approximately 94% of the bacterial flora associated with SBP. Initial treatment with cefotaxime (or ceftriaxone, if cefotaxime is unavailable) at a dose of 2 g IV q8h for seven days is recommended, unless or until culture results identify susceptibility to a specific narrow-spectrum antibiotic.2,4,7,9 After completion of a total of seven days of IV antibiotics, it is cost effective and safe to transition clinically improved patients to an oral antibiotic for SBP prophylaxis (see discussion below).9
A repeat paracentesis performed 48 hours after treatment is initiated is useful in assessing response to treatment, defined as a ≥ 25% decrease in ascitic fluid PMN count.7
Even if the infection is successfully treated, one-third of patients who have an episode of SBP develop renal failure; those who experience a recurrence of SBP within one year have a 50% to 60% mortality rate.4,7,9,14 This is because infection causes vasodilation, with a concomitant decrease in blood volume and compensatory activation of renal vasoconstrictors, so renal function worsens.
A randomized controlled trial found that patients treated with IV cefotaxime with albumin in doses of 1.5 g/kg on day 1 and 1 g/kg on day 3 had significantly lower rates of AKI and mortality than patients who received cefotaxime alone.15 An albumin infusion, given in conjunction with antibiotics, improves blood volume, enhances renal perfusion, and has been shown to decrease mortality from SBP from 29% to 10%.2,12,16
CASE For small bowel obstruction, the patient was made NPO and a nasogastric tube was placed for 36 hours. During this time, he had a bowel movement, diet was advanced, and the obstruction resolved.
At the same time, the patient was started on IV cefotaxime 2 g q8h for treatment of SBP, along with IV albumin and fluids. Diuretics were withheld because of impaired kidney function.
Ascitic fluid was cultured, and Streptococcus viridans was identified. After consultation with the infectious disease service, broader-spectrum vancomycin was added to the patient’s IV antibiotic regimen 24 hours after admission while culture results finalized. Cultures were analyzed after three days and found to be susceptible to ceftriaxone, which was administered 2 g IV q24h for three days, for a total of seven days of IV antibiotics.
The patient’s AKI improved after treatment with IV antibiotics, fluids, and albumin. Seventy-two hours after admission, repeat paracentesis revealed a significant decrease in white blood cells; cultures were negative for infection.
Neutrocytic ascites
In some cases, the bacteria count in ascitic fluid is so low that cultures may be negative.4 This is termed neutrocytic ascites, characterized by an elevated PMN cell count with negative cultures. Patients with neutrocytic ascites should be treated as though they have SBP.2,7
Prophylactic antibiotics
Of patients successfully treated for SBP, 69% will experience another episode within one year; many of these cases can be prevented with the use of prophylactic antibiotics. Historically, quinolones were used for SBP prophylaxis, and they are cost-effective. However, multiorganism drug resistance hasoccurred after quinolone prophylaxis, especially if given long-term.2,4,7
Alternatives to quinolones include trimethoprim/sulfamethoxazole double strength or ciprofloxacin 500 mg/d.2 Renal dose adjustment is necessary for patients with acute or chronic kidney disease and can usually be guided by a pharmacist. One study found norfloxacin and trimethoprim-sulfamethoxazole to be similarly effective for SBP prevention, but trimethoprim-sulfamethoxazole was noted to be more cost-effective.10
In patients with active gastrointestinal bleeding, IV ceftriaxone 1 g/d for seven days can be given for SBP prevention. In patients with low ascitic protein and/or history of prior SBP, norfloxacin 400 mg/d is an option.
Continue for conclusion >>
CASE On day 4 of hospitalization, the patient was determined to be stable for discharge, with close follow-up. After completion of three days of IV antibiotics on an outpatient basis, the patient began ciprofloxacin (500 mg/d) for SBP prophylaxis. His status on the transplant list was reactivated and, two weeks later, the patient received a liver transplant.
CONCLUSION
As the number of patients living with cirrhosis increases, it is very important for health care providers to appropriately identify and treat this patient population. Ascites and SBP can be deadly but can also be treated effectively. Ideal management includes early identification, proper laboratory testing and radiologic imaging, treatment with fluids and albumin, antibiotic administration in the acute care setting, and antibiotics for SBP prophylaxis. Diuretics should be used with caution and doses adjusted based on clinical judgment and the patient’s presentation. Patients should be referred for liver transplant evaluation. Early identification and treatment of ascites and SBP are essential for patient survival.
REFERENCES
1. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375-382.
2. Runyon BA. American Association for the Study of Liver Diseases practice guideline. Management of adult patients with ascites due to cirrhosis: update 2012. www.aasld.org/sites/default/files/guideline_documents/adultascitesenhanced.pdf. Accessed March 24, 2015.
3. European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397-417.
4. Sargent S. The management and nursing care of cirrhotic ascites. Br J Nurs. 2006;15(4):212-219.
5. Sargent S. Management of patients with advanced liver cirrhosis. Nurs Stand. 2006;21(11):48-56.
6. Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. American Association for the Study of Liver Diseases and the American Society of Transplantation. Evaluation for liver transplantation in adults: 2013 practice guideline. Hepatology. 2014;59(3):1144-65.
7. Loo NM, Souza FF, Garcia-Tsao G. Non-hemorrhagic acute complications associated with cirrhosis and portal hypertension. Best Pract Res Clin Gastroenterol. 2013;27(5):665-678.
8. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.
9. Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):
77-93.
10. Lontos S, Gow PJ, Vaughan RB, Angus PW. Norfloxacin and trimethoprim-sulfamethoxazole therapy have similar efficacy in prevention of spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2008;23(2): 252-255.
11. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36.
12. Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World J Hepatol. 2013;5(5):251-263.
13. Bone RC, Balk RA, Cerra FB, et al; ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655.
14. Wong F, O’Leary JG, Reddy KR, et al; North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6):1280-1288.
15. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409.
16. Rozga J, Piatek T, Malkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205-217.
Cirrhosis and its complications are among the top 10 causes of death in the United States.1,2 One of the most common complications of cirrhosis is ascites, an abnormal accumulation of fluid in the peritoneal cavity.3 Although ascites can be of nonhepatic origin, in approximately 85% of cases, the cause is cirrhosis.2,4
Developing in some 60% of cirrhosis patients within 10 years,3 ascites indicates disease progression from compensated to decompensated cirrhosis.5 Mortality from ascites is approximately 15% in the first year and 44% by the fifth year, so referral for liver transplant evaluation is often indicated.2
Frequently, however, patients do not meet the criteria for transplantation because of comorbidities such as morbid obesity, severe cardiac or pulmonary disease, severe malignancy, chemical dependency, or lack of caregiver support.6 Primary care clinicians need to know about the management of ascites in chronic liver disease in order to meet the significant ongoing health care needs of these patients.
This article reviews the diagnosis and treatment of cirrhosis-related ascites and discusses one particularly life-threatening infection—spontaneous bacterial peritonitis (SBP)—to which these patients are susceptible.
CASE A 52-year-old African-American man presented to the emergency department (ED) with complaints of severe diffuse abdominal pain, worsening over the past few days, as well as nausea and vomiting. History was significant for hepatitis C–related cirrhosis, unresponsive to antiviral treatment, and liver disease complications that included hepatic encephalopathy; portal hypertension; ascites requiring large-volume paracentesis every one to two weeks (most recently, six days earlier); esophageal varices (status postbanding by esophagogastroduodenoscopy); portal hypertensive gastropathy; and gastric varices.
Due to decompensated cirrhosis, the patient had previously undergone extensive screening, radiologic imaging, and laboratory testing, and was found by a multidisciplinary selection committee to be an acceptable liver transplant candidate. He was actively listed for transplantation.
Other significant history included hypertension; sleep apnea; gastroesophageal reflux; osteopenia; zinc, vitamin A, and vitamin D deficiencies; thrombocytopenia; and anemia of chronic disease (liver disease–related). Surgical history was negative. The patient reported no known drug allergies and was taking spironolactone (50 mg/d) and furosemide (20 mg/d).
Physical exam was notable for a low-grade fever of 99.1ºF; blood pressure, 132/81 mm Hg; heart rate, 84 beats/min; icteric sclerae; and moderate distress related to the patient’s abdominal pain, which worsened with deep palpation. The abdomen was distended, with ascites present as indicated by a positive fluid wave test. Bowel sounds were hypoactive. The patient was alert and oriented without asterixis; mental state was within normal limits.
Continue for pathophysiology of ascites >>
PATHOPHYSIOLOGY OF ASCITES
In a patient with cirrhosis, blood flow is reduced through the scarred liver and becomes retrograde to the normal flow pattern, causing portal hypertension. Portal hypertension causes vasodilators, such as nitric oxide, to be produced, leading to vasodilation of the splanchnic arterial system. Eventually, as vasodilation increases, the arterial receptors sense a decreased amount of blood flow in this part of the circulation. The body activates various systems (sympathetic nervous, antidiuretic hormone, and renin-angiotensin-aldosterone), resulting in increased water and sodium absorption and renal vasoconstriction. In turn, intestinal permeability and pressure in the capillaries respond, allowing fluid—ascites—to move into the peritoneal cavity.4,5,7
SPONTANEOUS BACTERIAL PERITONITIS
Developing in approximately 25% of patients with cirrhosis and ascites,8 spontaneous bacterial peritonitis (SBP) is thought to occur by translocation of intestinal bacteria moving through the mesenteric lymph nodes into the bloodstream and other body fluids (eg, ascites).4,7,9Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumonia are the bacteria most often responsible for SBP.2
If left untreated or treated too late, SBP can eventually lead to sepsis and septic shock.4,7,9 About 30% of cirrhosis patients with SBP will die of it or related complications;8 the one-year survival rate is 30% to 50% and the two-year survival rate, 25% to 30%.9 Mortality is increased by up to 50% in hospitalized patients with SBP.3,10,11
DIAGNOSIS
Ascites
In patients with cirrhosis, typical signs and symptoms of ascites include weight gain, increased abdominal girth and fullness, dullness to abdominal percussion, peripheral edema, and a positive fluid wave test.2,4
In both inpatient and outpatient settings, ascites should be sampled by diagnostic abdominal paracentesis (see Figure), which requires 30 to 50 mL of fluid.12 Laboratory analysis should include white blood cell count with differential, serum-ascites albumin gradient (SAAG), and total protein. If infection is suspected, samples should be sent for culture, using blood culture bottles, as well as for gram staining.2,12
SAAG is calculated by measuring the albumin concentration in ascitic fluid and serum specimens taken on the same day and then subtracting the ascitic fluid value from the serum value. With 97% accuracy, a SAAG ≥ 1.1 g/dL indicates portal hypertension, meaning that the ascites is likely hepatic in origin.2,12
Continue for SBP >>
SBP
Approximately 87% of patients with SBP will have signs or symptoms of infection,3 but symptoms can be very vague, so careful attention to detail and thorough assessment are necessary (see Table 1).2,4,7,12 An elevated absolute polymorphonuclear (PMN) leukocyte cell count of ≥ 250 cells/µL and a positive ascitic culture, with no other apparent source of intra-abdominal infection, are diagnostic for SBP.2
Any cirrhotic patient with ascites who presents with two or more of the following criteria for systemic inflammatory response syndrome should be evaluated for an infectious cause:13
• Temperature > 100.4°F or < 96.8°F
• Heart rate > 90 beats/min
• Tachypnea (respiratory rate > 20 breaths/min) or hyperventilation (arterial carbon dioxide tension [PaCO2] < 32 mm Hg)
• Abnormal white blood cell count (> 12,000/µL or < 4,000/µL or > 10% immature neutrophils [band forms])
CASE Abdominal x-ray, CT, and laboratory testing were ordered in the ED (see Table 2 for laboratory test results). Imaging revealed multiple loops of small bowel in the midabdomen, with several air-fluid levels and a paucity of gas in the rectum. These findings were consistent with small bowel obstruction. In addition, laboratory data were significant for leukocytosis (higher than the patient’s usual level), worsening hyperbilirubinemia and hypoalbuminemia, elevated serum creatinine level indicating acute kidney injury (AKI), and lactic acidosis. The patient was admitted to the hospital for further management.
Because of his severe abdominal pain and leukocytosis, bedside paracentesis was performed. He was found to have an absolute PMN cell count of approximately 1,273 cells/µL, suggesting a diagnosis of SBP. The patient’s status on the transplant list was changed to inactive due to the infection.
Next page: Treatment >>
TREATMENT
Ascites
First-line treatment for patients with ascites includes dietary sodium restriction (maximum 2,000 mg/d) and oral diuretics (spironolactone with or without furosemide).2,5 This regimen is effective for 90% of patients.5 Typically, a ratio of 100 mg of spironolactone to 40 mg of furosemide is ideal to promote adequate diuresis while maintaining normal electrolyte balance. Doses can be increased every three to five days, with maximum doses of 400 mg/d of spironolactone and 160 mg/d of furosemide.2 Renal function and sodium levels should be monitored closely and diuretic dosing adjusted based on clinical presentation to avoid volume depletion, which puts patients at risk for AKI and hyponatremia.5,12 Diuretics should not be given in cases of AKI (creatinine level > 2 mg/dL), kidney failure (ie, patients on dialysis), acute infection, uncontrolled encephalopathy, or severe hyponatremia (sodium < 120 mEq/L).2,4
Refractory ascites
Approximately 10% of patients develop refractory ascites when the condition becomes unresponsive to diuretics or when adverse effects preclude the use of diuretics.5 In such cases, large-volume paracentesis (> 5 L removed) is the standard treatment. The patient should be monitored closely during the procedure; blood pressure can decrease drastically due to the large fluid loss. Vital signs should be checked every 15 min to 30 min for the first hour postprocedure, and then hourly if signs remain stable. Albumin should be administered (6-8 g/L of fluid removed) to increase circulating fluid volume.2 The patient’s renal function (ie, serum creatinine level and urinary output) should also be monitored closely, as dehydration and/or AKI can occur.4 Complications of paracentesis are uncommon but may include bleeding, infection, and bowel perforation. No data support the routine administration of blood products before paracentesis.2
Treatment of SBP
Broad-spectrum antibiotics, particularly cefotaxime, are effective against approximately 94% of the bacterial flora associated with SBP. Initial treatment with cefotaxime (or ceftriaxone, if cefotaxime is unavailable) at a dose of 2 g IV q8h for seven days is recommended, unless or until culture results identify susceptibility to a specific narrow-spectrum antibiotic.2,4,7,9 After completion of a total of seven days of IV antibiotics, it is cost effective and safe to transition clinically improved patients to an oral antibiotic for SBP prophylaxis (see discussion below).9
A repeat paracentesis performed 48 hours after treatment is initiated is useful in assessing response to treatment, defined as a ≥ 25% decrease in ascitic fluid PMN count.7
Even if the infection is successfully treated, one-third of patients who have an episode of SBP develop renal failure; those who experience a recurrence of SBP within one year have a 50% to 60% mortality rate.4,7,9,14 This is because infection causes vasodilation, with a concomitant decrease in blood volume and compensatory activation of renal vasoconstrictors, so renal function worsens.
A randomized controlled trial found that patients treated with IV cefotaxime with albumin in doses of 1.5 g/kg on day 1 and 1 g/kg on day 3 had significantly lower rates of AKI and mortality than patients who received cefotaxime alone.15 An albumin infusion, given in conjunction with antibiotics, improves blood volume, enhances renal perfusion, and has been shown to decrease mortality from SBP from 29% to 10%.2,12,16
CASE For small bowel obstruction, the patient was made NPO and a nasogastric tube was placed for 36 hours. During this time, he had a bowel movement, diet was advanced, and the obstruction resolved.
At the same time, the patient was started on IV cefotaxime 2 g q8h for treatment of SBP, along with IV albumin and fluids. Diuretics were withheld because of impaired kidney function.
Ascitic fluid was cultured, and Streptococcus viridans was identified. After consultation with the infectious disease service, broader-spectrum vancomycin was added to the patient’s IV antibiotic regimen 24 hours after admission while culture results finalized. Cultures were analyzed after three days and found to be susceptible to ceftriaxone, which was administered 2 g IV q24h for three days, for a total of seven days of IV antibiotics.
The patient’s AKI improved after treatment with IV antibiotics, fluids, and albumin. Seventy-two hours after admission, repeat paracentesis revealed a significant decrease in white blood cells; cultures were negative for infection.
Neutrocytic ascites
In some cases, the bacteria count in ascitic fluid is so low that cultures may be negative.4 This is termed neutrocytic ascites, characterized by an elevated PMN cell count with negative cultures. Patients with neutrocytic ascites should be treated as though they have SBP.2,7
Prophylactic antibiotics
Of patients successfully treated for SBP, 69% will experience another episode within one year; many of these cases can be prevented with the use of prophylactic antibiotics. Historically, quinolones were used for SBP prophylaxis, and they are cost-effective. However, multiorganism drug resistance hasoccurred after quinolone prophylaxis, especially if given long-term.2,4,7
Alternatives to quinolones include trimethoprim/sulfamethoxazole double strength or ciprofloxacin 500 mg/d.2 Renal dose adjustment is necessary for patients with acute or chronic kidney disease and can usually be guided by a pharmacist. One study found norfloxacin and trimethoprim-sulfamethoxazole to be similarly effective for SBP prevention, but trimethoprim-sulfamethoxazole was noted to be more cost-effective.10
In patients with active gastrointestinal bleeding, IV ceftriaxone 1 g/d for seven days can be given for SBP prevention. In patients with low ascitic protein and/or history of prior SBP, norfloxacin 400 mg/d is an option.
Continue for conclusion >>
CASE On day 4 of hospitalization, the patient was determined to be stable for discharge, with close follow-up. After completion of three days of IV antibiotics on an outpatient basis, the patient began ciprofloxacin (500 mg/d) for SBP prophylaxis. His status on the transplant list was reactivated and, two weeks later, the patient received a liver transplant.
CONCLUSION
As the number of patients living with cirrhosis increases, it is very important for health care providers to appropriately identify and treat this patient population. Ascites and SBP can be deadly but can also be treated effectively. Ideal management includes early identification, proper laboratory testing and radiologic imaging, treatment with fluids and albumin, antibiotic administration in the acute care setting, and antibiotics for SBP prophylaxis. Diuretics should be used with caution and doses adjusted based on clinical judgment and the patient’s presentation. Patients should be referred for liver transplant evaluation. Early identification and treatment of ascites and SBP are essential for patient survival.
REFERENCES
1. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375-382.
2. Runyon BA. American Association for the Study of Liver Diseases practice guideline. Management of adult patients with ascites due to cirrhosis: update 2012. www.aasld.org/sites/default/files/guideline_documents/adultascitesenhanced.pdf. Accessed March 24, 2015.
3. European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397-417.
4. Sargent S. The management and nursing care of cirrhotic ascites. Br J Nurs. 2006;15(4):212-219.
5. Sargent S. Management of patients with advanced liver cirrhosis. Nurs Stand. 2006;21(11):48-56.
6. Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. American Association for the Study of Liver Diseases and the American Society of Transplantation. Evaluation for liver transplantation in adults: 2013 practice guideline. Hepatology. 2014;59(3):1144-65.
7. Loo NM, Souza FF, Garcia-Tsao G. Non-hemorrhagic acute complications associated with cirrhosis and portal hypertension. Best Pract Res Clin Gastroenterol. 2013;27(5):665-678.
8. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.
9. Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):
77-93.
10. Lontos S, Gow PJ, Vaughan RB, Angus PW. Norfloxacin and trimethoprim-sulfamethoxazole therapy have similar efficacy in prevention of spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2008;23(2): 252-255.
11. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36.
12. Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World J Hepatol. 2013;5(5):251-263.
13. Bone RC, Balk RA, Cerra FB, et al; ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655.
14. Wong F, O’Leary JG, Reddy KR, et al; North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6):1280-1288.
15. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409.
16. Rozga J, Piatek T, Malkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205-217.