User login
Introduction
For decades, virtually all injury was treated with open operative surgery. Resuscitation was based on the belief that large-volume crystalloid infusion to raise blood pressure (BP) to normal was the optimal therapy. Advanced trauma life support teaching was that 2 L of crystalloid fluid should be the initial resuscitation for all trauma patients, and those who failed to respond should receive additional crystalloid fluid. Patients did not receive a blood transfusion until later in treatment, and fresh frozen plasma (FFP) and platelets were not given until 10 U of blood had been administered. Regardless of the fluid infused, the goal of initial resuscitation was to raise BP to a normal level. During the time I (TS) was chair of the emergency medicine department at the State University of New York’s Kings County Hospital, I remember administering liters of crystalloid fluid preoperatively, believing it was not safe to operate until the patient had been what we termed “adequately resuscitated.”
However, as early as 1918, Walter B. Cannon, MD, correctly observed that fluid therapy without hemostasis was not wise, and numerous animal studies since then also raised serious questions about this approach. This article points out the revolutionary changes in the thinking and practice of resuscitation that have occurred in the last 20 years. We now realize that raising BP to normal only perpetuates hemorrhage. Hypotension treated with additional volume resuscitation without surgical control of hemorrhage creates a cycle leading to dilution of clotting factors and red blood cells (RBCs), recurrent hypotension, and ultimately death.
The realization that early blood transfusion is probably the wisest course is a concept that has only been in clinical practice for less than 15 years. Major trauma centers now routinely keep type O negative blood in the ED refrigerators so that it is instantly available.
Our understanding of trauma coagulopathy also has changed dramatically. Once thought to be simply a consequence of hypotension and hypothermia, we now realize that coagulopathy following trauma is far more complicated and likely occurs in concert with the inflammatory response to serious injury. Regardless of its etiology, we have recognized that earlier administration of plasma and platelets following trauma prevents coagulopathy, and this approach is more beneficial than treating coagulopathy after it develops. There has been much debate about the optimal ratios of RBCs, plasma, and platelets, and the ideal ratio has yet to be determined. The idea that “one-size-fits-all” is almost certainly not the case: Different patients require different and more precise treatment strategies.
For years, we have relied on laboratory measurements of coagulation to guide transfusion therapy, but standard laboratory values often take over 30 minutes to obtain. In an extremely dynamic situation involving large-volume blood loss, this interval is too lax. A more personalized approach using rapidly available technology, such as thromboelastography (TEG), allows for real-time assessment of a multiplicity of coagulation dynamics and rapid correction of any abnormalities. Procoagulants such as factor 7A, prothrombin complex concentrate (PCC), and tranexamic acid (TXA) have a role. However, the data to support the use of these expensive agents is lacking. While they certainly can be life-saving, each of these components brings with it a risk of causing indiscriminate coagulation—even in areas of the body that are not injured. Moreover, their availability in nontrauma centers is either limited or not an option.
There is little question that our rapid advances in understanding resuscitation and transfusion practice has saved lives. Twenty years ago, intensive care units were populated by trauma patients who had received many liters of crystalloid fluid, and at least partly a consequence of the resuscitation experience, many had severe respiratory failure. Open abdomens were common and also a likely consequence of large-volume crystalloid use. While these problems have not entirely disappeared, they now occur much less frequently.
Standardizing trauma care has also helped enhance patient care a great deal. Most major trauma centers have a “massive transfusion” protocol which allows the blood bank to prepare coolers containing not only blood, but also plasma, platelets, and procoagulants. This practice obviates the need to order the components individually. Rapid access to technology such as TEG allows emergency physicians (EPs) and other trauma care professionals to precisely guide transfusion therapy, but this remains an area of intensely debated investigation. Hopefully, our understanding will continue to mature over the next few years.
Another area of trauma care that has rapidly evolved is the use of endovascular techniques for trauma hemostasis. The realization that we can obtain control of vascular injury without the need for a large open operation has revolutionized care. While endovascular techniques have been used for pelvic hemostasis since 1972, we now use it regularly in every body cavity. Splenic artery embolization was developed by our (TS) group in Brooklyn, New York in 1995, and its use has now expanded to other abdominal solid organ injuries.
Injuries to the thoracic aorta once required a thoracotomy, cardiopulmonary bypass, and open repair. Stent grafting is now the treatment of choice for these injuries, allowing for a minimally invasive solution, and permitting those with both aortic and many other injuries to receive care for all of these wounds much sooner than was possible in the past, when multiply injured patients were simply not considered candidates for early open repair.
Thoracotomy in the ED has been widely practiced for a variety of indications. While it is still the only available solution for injury to the heart and/or proximal pulmonary vasculature in a patient who is hemodynamically unstable and/or in extremis, other options now exist to obtain vascular inflow for patients bleeding in the abdomen or pelvis. The use of transfemoral balloons for aortic occlusion allows clinicians to temporize hemorrhage without a huge open operation, and resuscitative endovascular balloon occlusion of the aorta (REBOA), has only been available for the last several years. The exact indications, wisest strategy, length of time the balloon can be inflated, rate of complications, and who is the appropriate physician (eg, EP, intensivist, vascular surgeon) to insert it, all remain questions requiring resolution. Much more work is necessary to pursue the role that REBOA can have in the care of desperately injured trauma patients.
There has been a revolution in the care of severely injured patients. After 50 years of thinking that we knew the answers, we have come to realize that those answers were wrong. Newer resuscitation strategies, as well as new treatment strategies continue to evolve, allowing us to refine care of severely injured patients. Perhaps the one thing we have really learned is that we do not have all of the answers and that the discussion must continue if we are to do better at serving more trauma victims.
Damage Control Resuscitation
In the United States, trauma is the leading cause of death in patients younger than age 45 years and ranks as the fifth leading cause of death among all age groups. Hemorrhage remains the leading cause of preventable death in the trauma population,1 and one of the most important recent changes in our care of the injured patient is the manner in which we manage hemorrhage. As noted earlier, there has been a paradigm shift away from large-volume crystalloid resuscitation and toward what has been termed “damage control resuscitation” (DCR).2,3
The principles of the DCR strategy are aimed at preemptively treating the lethal triad of hypothermia, acidosis, and coagulopathy in conjunction with control of surgical bleeding using damage control surgery. The main principles of DCR include “permissive hypotension,” prevention of heat loss and/or active warming, minimizing the use of crystalloid infusions, and initiating resuscitation with blood products in a ratio that more closely resembles whole blood.2
Permissive Hypotension
Permissive hypotension, also referred to as hypotensive resuscitation, is not considered a goal or an endpoint, but rather a “bridge” to definitive surgical control of hemorrhage. The body’s initial response to injury involves vasoconstriction and early clot formation, a process facilitated by hypotension. The rationale for permissive hypotension is that attempting to drive the BP up to normal ranges may interfere with vasoconstriction, as well as physically disrupting this early clot, leading to increased bleeding and further hypotension.
This concept has been corroborated by many animal and human studies.3 In 1994, the landmark study by Bickell et al4 randomized patients with penetrating torso trauma and a systolic BP (SBP) of 90 mm Hg or lower to either immediate or delayed fluid resuscitation. Their study demonstrated that patients whose fluid resuscitation was delayed until they reached the operating room had improved outcomes. The study supported the long-time prehospital practice of the “scoop-and-run” strategy, especially in penetrating torso trauma.
In 2003, Sondeen et al5 used a swine model of aortic injury to find an inflection point for clot disruption and re-bleeding during volume resuscitation. They found the inflection point to be a mean arterial pressure (MAP) of 64 mm Hg and an SBP of 94 mm Hg, regardless of the size of the aortotomy. Using an animal model of hemorrhagic shock, Li et al6 demonstrated in 2011 that resuscitation to a MAP of 50 mm Hg was associated with a decreased amount of blood loss as well as with improved survival compared to patients who were resuscitated to a MAP of 80 mm Hg. However, they also showed that after a time period of more than 90 to 120 minutes, the lower MAP group had increased end organ damage and worse outcomes, emphasizing the importance of prompt surgical control of bleeding—regardless of preoperative resuscitation strategy.
Other studies, though, have not shown a clear benefit to permissive hypotension. A 2002 study by Dutton et al7 showed that titration of initial fluid to a lower SBP (70 mm Hg) did not affect mortality when compared to a target resuscitation MAP of more than 100 mm Hg. Further, in 2014, a plenary paper presented to the American Association for the Surgery of Trauma demonstrated that controlled resuscitation (CR) strategy was safe and feasible,8 but did not demonstrate a mortality benefit in the overall cohort, though patients with blunt trauma who received CR had improved survival at 24 hours.
The group at Ben Taub General Hospital in Houston, Texas recently performed a randomized controlled trial evaluating intraoperative hypotensive resuscitation strategies. Patients in hemorrhagic shock were randomized to either an intraoperative MAP goal of 50 mm Hg or 65 mm Hg.9,10 Preliminary results suggested that targeting a lower MAP resulted in fewer blood product transfusions, less fluid administration, less coagulopathy, and lower mortality in the early postoperative period. Additionally, they demonstrated a nonsignificant trend toward improved 30-day mortality in the lower MAP group.9 Moreover, in this study there was no increased morbidity associated with the hypotensive strategy,10 suggesting that the approach was safe. Unfortunately, the trial was stopped early due to slow enrollment.
Despite the overall promising results with permissive hypotension, it is important to remember that it is contraindicated in patients with known or suspected traumatic brain injury, as hypotension has been shown to be detrimental in this population.11
Hemostatic Resuscitation and Coagulopathy
Avoiding Aggressive Crystalloid Resuscitation. While the ideal MAP to target during DCR remains unclear, the potential harm caused by aggressive crystalloid resuscitation has become more evident. Infusing excessive amounts of crystalloid has been shown to be associated with increased ventilator days, multisystem organ failure, abdominal compartment syndrome, and surgical-site infections12—all of which have also been associated with systemic consequences of increased inflammation, including increased release of tumor necrosis factor-alpha and other proinflammatory cytokines.13
Rodent studies have demonstrated large-volume crystalloid administration and breakdown or “thinning” of the endothelial glycocalyx, which leads to increased capillary leak, third-spacing, and ultimately intravascular volume depletion.14,15 This mechanism has been linked to pulmonary complications, namely acute lung injury and acute respiratory distress syndrome. Enteric edema resulting from aggressive crystalloid resuscitation has also been associated with prolonged postoperative ileus, increased risk of anastomotic leak,13 and inability to achieve primary fascial closure.16 All of the aforementioned complications are reduced when employing a restrictive fluid resuscitation strategy.17
Aggressive crystalloid administration in hemorrhagic shock also leads to dilutional coagulopathy. Multiple animal and human studies have shown an association between increased crystalloid volumes in hemorrhaging patients and increasing coagulopathy, blood loss, and mortality. In 2004, Barak et al18 demonstrated that administration of a high volume of crystalloid fluid (>3 L) or colloid (500 mL) was associated with postoperative coagulopathy; whereas in 2017, Harada et al,19 at Cedars-Sinai Medical Center in New York, demonstrated over a 10-year period that decreased high-volume (>2 L) crystalloid resuscitation paralleled a decrease in mortality.
Massive Transfusion Protocols. Many trauma centers have shifted away from high-volume crystalloid resuscitation in favor of massive transfusion protocols (MTPs) utilizing standardized ratios that more closely mimic whole blood. The MTPs center on the principle of equal transfusion ratios of blood product as opposed to packed RBCs (PRBCs) alone. This means effecting a plasma-rich resuscitation and preemptive correction of coagulopathy with FFP and platelets in addition to PRBCs.
Data from a US Army combat support hospital have demonstrated improved survival with an FFP to PRBC ratio of more than 1:1.4,20 and civilian studies have produced similar findings.21-23 All of these studies also noted improved mortality with higher (>1:2) platelet to PRBC ratios.22,23 Although, the ideal ratio remains unknown, many MTPs aim for 1:1:1 ratio (6 U FFP to 6 packed platelets to 6 U PRBC), which most closely mimics whole blood.
The Pragmatic Randomized Optimal Platelet and Plasma Ratios trial was a recent large multicenter randomized trial that compared transfusion ratios of 1:1:1 and 1:1:2. The trial was unable to demonstrate a difference in mortality at either 24 hours or 30 days, though more patients in the 1:1:1 ratio group achieved hemostasis and fewer patients in this group died from exsanguination in the first 24 hours.24Prehospital PRBC Administration. A number of studies have looked at prehospital administration of PRBCs.25-27 Holcomb et al25 showed no overall survival advantage at 24 hours, but did demonstrate a negligible blood-product wastage. In 2015 Brown et al26 found an increase probability of 24-hour survival, decreased shock, and lower 24-hour PRBC requirements with pretrauma-center PRBC transfusion. That same year Brown et al27 demonstrated that prehospital PRBC transfusion in severely injured blunt trauma patients was associated with decreased 24-hour and 30-day mortality rates, and a lower risk of coagulopathy. Currently, the Prehospital Air Medical Plasma trial is enrolling patients to evaluate the prehospital administration of plasma.28 The primary endpoint of the study is 30-day mortality; the tentative completion date for the study is January 2018.
Tranexamic Acid. Another important development in the treatment of hemorrhagic shock in recent years has been the use of TXA, an antifibrinolytic agent which inhibits the conversion of plasminogen to plasmin. It has been shown to decrease mortality in both civilian and military trauma populations.29,30
The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 trial was a large multicenter randomized trial, which showed a survival benefit among those who received TXA. The generalizability of the study has been questioned in the setting of modern urban trauma centers, as most of those enrolled in the study were from hospitals with no formal MTPs and a limited availability of blood products. Additionally, no laboratory measures of fibrinolysis were available.30
Most experts currently recommend TXA use as part of an MTP if there is evidence of hyperfibrinolysis on TEG or in severe hemorrhagic shock when the time from injury has been less than 3 hours, as studies have shown increased mortality when TXA was administered longer than 3 hours after injury.30
Viscoelastic Assays
An alternative approach to standardized ratio MTPs involves goal-directed hemostatic resuscitation using viscoelastic assays to guide transfusion of blood-product components. Both TEG and rotational thromboelastometry (ROTEM) are point-of-care tools for assessment of coagulation parameters of whole blood. Although they are not new technology, their use in trauma resuscitation is a relatively new concept.
While ROTEM is more commonly used in Europe, TEG is more popular and commonly used in the United States, though not exclusively.31,32
Thromboelastography
The TEG parameters most commonly used clinically are reaction time (R-time), kinetics time, angle, maximum amplitude (MA), and lysis at 30 minutes (LY30).
Reaction Time. The R-time is measured in minutes and represents the time to clot initiation, reflecting activity of coagulation factors. It is used in TEG-guided MTPs to trigger transfusion of FFP.31,32 The R-time is measured at the time the clot strength reaches an amplitude of 2 mm.31 The angle reflects the rate of rise of the amplitude of the TEG tracing, or the rate of increase in clot strength. Clinically, the angle represents fibrinogen concentration and function, and is used to trigger transfusion of cryoprecipitate or fibrinogen concentrate in MTPs.31,32
Maximum Amplitude. The MA is reached by the TEG tracing, or the maximum clot strength achieved. Although the MA has been shown to correlate with platelet count, it actually represents platelet count and function as well as fibrinogen activity, all of which contribute to clot strength. Clinically, MA is used to trigger platelet transfusion and/or administration of desmopressin in MTPs.31,32
Lysis at 30 Minutes. The LY30 is defined as the percent reduction in clot strength 30 minutes after reaching MA.31,32 Normal LY30 values are between 0% and 7.5%; however, these values have been challenged in recent studies, which have reported that an LY30 greater than 3% (termed hyperfibrinolysis) confers a significant increase in mortality and an increased likelihood of requiring massive transfusion.31,33 These findings have led to incorporation of this lower threshold as a trigger for administration of TXA during MTPs. Furthermore, an LY30 of less than 0.8% (described as fibrinolysis shutdown) has also been found to confer an increase in mortality,34 which has led many to advocate for goal-directed administration of TXA, rather than empiric administration, as these patients are more likely to be harmed than helped by such an intervention.31
Rapid Thromboelastography
Rapid TEG employs tissue factor to accelerate clot initiation and reaction time, providing an additional parameter which reflects coagulation factor activity: the activated clotting time (ACT).32
Activated Clotting Time. Historically used in cardiac surgery to measure anticoagulation during a cardiopulmonary bypass, ACT represents the same phase of coagulation as R-time, but is measured in seconds instead of minutes.31 The ACT has been found to correlate with prothrombin time/international normalized ratio (PT/INR), and accurately predicts the need for MTP.
Cotton et al35 found that an ACT of more than 128 seconds predicted patients requiring MTP, and an ACT lower than 105 seconds predicted those who required no transfusions in the first 24 hours after injury.35
The ACT can be used to trigger transfusion of FFP, but at certain thresholds, may also be used to trigger the early transfusion of cryoprecipitate and platelets.36 Moore et al36 found that an ACT over 140 seconds was able to predict an abnormal angle and MA. This had led to using this threshold as a trigger for early administration of cryoprecipitate and platelets, given this parameter is available within 5 minutes—long before the angle and MA have resulted.
Efficacy
The use of a TEG-guided strategy for MTP in trauma has shown great promise. In 2013, Tapia et al37 compared a historical cohort who received 1:1:1 MTP to a TEG-guided MTP and demonstrated improved mortality.In 2016, Gonzalez et al38 compared TEG-guided transfusion vs conventional coagulation tests (PT/INR, PTT, fibrinogen, platelets). The authors found a significant decrease in mortality and platelet and FFP transfusion when TEG-guided resuscitation is used.
Endovascular Techniques
The use of endovascular techniques in trauma continues to evolve. According to the National Trauma Data Bank, the use of endovascular therapies has increased from 1% of trauma cases in 2002 to 11% in 2008.39
Thoracic Endovascular Aortic Repair
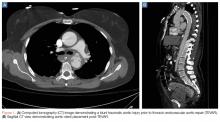
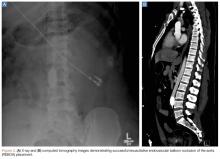
Thoracic endovascular aortic repair (TEVAR) for blunt thoracic aortic injury has essentially replaced open surgical repair. (See Figures 1a and 1b for an example of a blunt traumatic aortic injury prior to and post-TEVAR placement.)
Transarterial Catheter Embolization
Endovascular treatments have also been used successfully in the management of injuries to aortic branch vessels and extremity vessels.42 Transarterial catheter embolization with coils, plugs, or gel foam is being employed with increasing frequency to achieve hemostasis in the pelvis and spleen.42 It may also be used as an adjunct to laparotomy and perihepatic packing in high-grade liver injuries, though it is associated with significant morbidity related to hepatic necrosis, bile leaks, and abscesses.43,44
Resuscitative Endovascular Balloon Occlusion of the Aorta
Most recently, REBOA has been used for noncompressible torso hemorrhage following trauma. This method involves percutaneous arterial cannulation of the common femoral artery and advancement of a balloon into the aorta, where it is then inflated at the desired level.
Once inflated, the balloon obstructs arterial inflow to the area of hemorrhage, curtailing blood loss, and increases proximal BP, improving coronary and cerebral perfusion. Multiple case reports and case series have described successful use of REBOA for hemorrhage control, including prehospital use by physicians in the United Kingdom. The largest series to date looked at 114 patients, of whom 46 had REBOA placement and 68 had open aortic occlusion through resuscitative thoracotomy.45 Those treated with REBOA were significantly more likely to achieve hemodynamic stability (defined as SBP >90 mm Hg for >5 minutes). Furthermore, the authors noted minimal complications from REBOA and no difference in time to successful aortic occlusion, regardless of technique. There was also no difference in mortality between the two groups. Despite the small number of studies in trauma patients, REBOA has been established as a viable alternative to open aortic occlusion. The prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery registry established by the American Association for the Surgery of Trauma is continuing to enroll patients and will hopefully answer many of the current uncertainties regarding the use of REBOA.
Conclusion
Strategies and techniques for the care of the injured patient have changed significantly in the past few years. Damage control resuscitation includes three elements: damage control surgery, permissive hypotension, and blood-product resuscitation.
The goals of lowering MAP in hemorrhagic shock appear to be safe and make sense physiologically, but have yet to show clear mortality benefit. Avoidance of excessive crystalloid resuscitation and trends toward more physiological ratios of blood product resuscitation have shown better outcomes. While the ideal ratio of blood products in transfusion remains unknown, the use of a massive transfusion strategy is preferable to crystalloid fluids. The use of viscoelastic assays (TEG and ROTEM) have allowed for goal-directed blood product resuscitation and may improve outcomes when compared with prescribed resuscitation ratios.
Finally, endovascular techniques in trauma have been increasingly utilized over the past 15 years, making nonoperative management with angiographic embolization for solid organ injury common practice now in most trauma centers worldwide. Temporary aortic balloon occlusion with REBOA appears promising in many cases of noncompressible truncal hemorrhage until definitive hemostasis can be achieved, but studies are needed to determine its ultimate place in the care of the trauma patient.
1. Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158-163. doi:10.1007/s00268-009-0266-1.
2. Bogert JN, Harvin JA, Cotton BA. Damage control resuscitation. J Intensive Care Med. 2016;31(3):177-186. doi:10.1177/0885066614558018.
3. Kaafarani HMA, Velmahos GC. Damage control resuscitation in trauma. Scand J Surg. 2014;103(2):81-88. doi:10.1177/1457496914524388.
4. Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105-1109. doi:10.1056/NEJM199410273311701.
5. Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110-S117. doi:10.1097/01.TA.0000047220.81795.3D.
6. Li T, Zhu Y, Hu Y, et al. Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology. 2011;114(1):111-119. doi:10.1097/ALN.0b013e3181fe3fe7.
7. Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52(6):1141-1146.
8. Schreiber MA, Meier EN, Tisherman SA, et al; ROC Investigators. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687-695. doi:10.1097/TA.0000000000000600.
9. Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652-663. doi:10.1097/TA.0b013e31820e77ea.
10. Carrick MM, Morrison CA, Tapia NM, et al. Intraoperative hypotensive resuscitation for patients undergoing laparotomy or thoracotomy for trauma: Early termination of a randomized prospective clinical trial. J Trauma Acute Care Surg. 2016;80(6):886-896. doi:10.1097/TA.0000000000001044.
11. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216-222.
12. Kasotakis G, Sideris A, Yang Y, et al. Inflammation and Host Response to Injury Investigators. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74(5):1215-1221; discussion 1221-1222. doi:10.1097/TA.0b013e3182826e13.
13. Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115-121. doi:10.1097/01.shk.0000209564.84822.f2.
14. Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289-1295. doi:10.1213/ANE.0b013e318210385c.
15. Torres LN, Sondeen JL, Ji L, Dubick MA, Filho IT. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759-766. doi:10.1097/TA.0b013e3182a92514.
16. Bradley M, Galvagno S, Dhanda A, et al. Damage control resuscitation protocol and the management of open abdomens in trauma patients. Am Surg. 2014;80(8):768-775.
17. Brandstrup B, Tønnesen H, Beier-Holgersen R, et al; Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641-648. doi:10.1097/01.sla.0000094387.50865.23.
18. Barak M, Rudin M, Vofsi O, Droyan A, Katz Y. Fluid administration during abdominal surgery influences on coagulation in the postoperative period. Curr Surg. 2004;61(5):459-462. doi:10.1016/j.cursur.2004.02.002.
19. Harada MY, Ko A, Barmparas G, et al. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int J Surg. 2017;38:78-82. doi:10.1016/j.ijsu.2016.12.073.
20. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805-813. doi:10.1097/TA.0b013e3181271ba3.
21. Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177-1782; discussion 1182-1183. doi:10.1097/TA.0b013e31816c5c80.
22. Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447-458. doi:10.1097/SLA.0b013e318185a9ad.
23. Holcomb JB, del Junco DJ, Fox EE, et al; PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127-136. doi:10.1001/2013.jamasurg.387.
24. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
25. Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care. 2015;19(1):1-9. doi:10.3109/10903127.2014.923077.
26. Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg. 2015;220(5):797-808. doi:10.1016/j.jamcollsurg.2015.01.006.
27. Brown JB, Cohen MJ, Minei JP, et al; Inflammation and the Host Response to Injury Investigators. Pretrauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured patients with blunt trauma. Ann Surg. 2015;261(5):997-1005. doi:10.1097/SLA.0000000000000674.
28. Brown JB, Guyette FX, Neal MD, et al. Taking the blood bank to the field: the design and rationale of the Prehospital Air Medical Plasma (PAMPer) trial. Prehosp Emerg Care. 2015;19(3):343-350. doi:10.3109/10903127.2014.995851.
29. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119. doi:10.1001/archsurg.2011.287.
30. Shakur H, Roberts I, Bautista R, et al; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi:10.1016/S0140-6736(10)60835-5.
31. Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119-134. doi:10.1016/j.ccc.2016.09.002.
32. Abdelfattah K, Cripps MW. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg. 2016;33(Pt B):196-201. doi:10.1016/j.ijsu.2015.09.036.
33. Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365-370; discussion 370. doi:10.1097/TA.0b013e31825c1234.
34. Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811-817. doi:10.1097/TA.0000000000000341.
35. Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71(2):407-414; discussion 414-417. doi:10.1097/TA.0b013e31821e1bf0.
36. Moore HB, Moore EE, Chin TL, et al. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014;156(3):564-569. doi:10.1016/j.surg.2014.04.017.
37. Tapia NM, Chang A, Norman M, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378-385; discussion 385-386. doi:10.1097/TA.0b013e31827e20e0.
38. Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051-1059. doi:10.1097/SLA.0000000000001608.
39. Avery LE, Stahlfeld KR, Corcos AC, et al. Evolving role of endovascular techniques for traumatic vascular injury: a changing landscape? J Trauma Acute Care Surg. 2012;72(1):41-46; discussion 46-47. doi:10.1097/TA.0b013e31823d0f03.
40. Demetriades D, Velmahos GC, Scalea TM, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma. 2008;64(6):1415-1418; discussion 1418-1419. doi:10.1097/TA.0b013e3181715e32.
41. Azizzadeh A, Ray HM, Dubose JJ, et al. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg. 2014;76(2):510-516. doi:10.1097/TA.0b013e3182aafe8c.
42. Brenner M, Hoehn M, Rasmussen TE. Endovascular therapy in trauma. Eur J Trauma Emerg Surg. 2014;40(6):671-678. doi:10.1007/s00068-014-0474-8.
43. Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma. 2009;66(3):621-627; discussion 627-629. doi:10.1097/TA.0b013e31819919f2.
44. Letoublon C, Morra I, Chen Y, Monnin V, Voirin D, Arvieux C. Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma. 2011;70(5):1032-1036; discussion 1036-1037. doi:10.1097/TA.0b013e31820e7ca1.
45. DuBose JJ, Scalea TM, Brenner M, et al; AORTA Study Group. The AAST prospective Aortic Occlusion for Resuscitati on in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419. doi:10.1097/TA.0000000000001079.
Introduction
For decades, virtually all injury was treated with open operative surgery. Resuscitation was based on the belief that large-volume crystalloid infusion to raise blood pressure (BP) to normal was the optimal therapy. Advanced trauma life support teaching was that 2 L of crystalloid fluid should be the initial resuscitation for all trauma patients, and those who failed to respond should receive additional crystalloid fluid. Patients did not receive a blood transfusion until later in treatment, and fresh frozen plasma (FFP) and platelets were not given until 10 U of blood had been administered. Regardless of the fluid infused, the goal of initial resuscitation was to raise BP to a normal level. During the time I (TS) was chair of the emergency medicine department at the State University of New York’s Kings County Hospital, I remember administering liters of crystalloid fluid preoperatively, believing it was not safe to operate until the patient had been what we termed “adequately resuscitated.”
However, as early as 1918, Walter B. Cannon, MD, correctly observed that fluid therapy without hemostasis was not wise, and numerous animal studies since then also raised serious questions about this approach. This article points out the revolutionary changes in the thinking and practice of resuscitation that have occurred in the last 20 years. We now realize that raising BP to normal only perpetuates hemorrhage. Hypotension treated with additional volume resuscitation without surgical control of hemorrhage creates a cycle leading to dilution of clotting factors and red blood cells (RBCs), recurrent hypotension, and ultimately death.
The realization that early blood transfusion is probably the wisest course is a concept that has only been in clinical practice for less than 15 years. Major trauma centers now routinely keep type O negative blood in the ED refrigerators so that it is instantly available.
Our understanding of trauma coagulopathy also has changed dramatically. Once thought to be simply a consequence of hypotension and hypothermia, we now realize that coagulopathy following trauma is far more complicated and likely occurs in concert with the inflammatory response to serious injury. Regardless of its etiology, we have recognized that earlier administration of plasma and platelets following trauma prevents coagulopathy, and this approach is more beneficial than treating coagulopathy after it develops. There has been much debate about the optimal ratios of RBCs, plasma, and platelets, and the ideal ratio has yet to be determined. The idea that “one-size-fits-all” is almost certainly not the case: Different patients require different and more precise treatment strategies.
For years, we have relied on laboratory measurements of coagulation to guide transfusion therapy, but standard laboratory values often take over 30 minutes to obtain. In an extremely dynamic situation involving large-volume blood loss, this interval is too lax. A more personalized approach using rapidly available technology, such as thromboelastography (TEG), allows for real-time assessment of a multiplicity of coagulation dynamics and rapid correction of any abnormalities. Procoagulants such as factor 7A, prothrombin complex concentrate (PCC), and tranexamic acid (TXA) have a role. However, the data to support the use of these expensive agents is lacking. While they certainly can be life-saving, each of these components brings with it a risk of causing indiscriminate coagulation—even in areas of the body that are not injured. Moreover, their availability in nontrauma centers is either limited or not an option.
There is little question that our rapid advances in understanding resuscitation and transfusion practice has saved lives. Twenty years ago, intensive care units were populated by trauma patients who had received many liters of crystalloid fluid, and at least partly a consequence of the resuscitation experience, many had severe respiratory failure. Open abdomens were common and also a likely consequence of large-volume crystalloid use. While these problems have not entirely disappeared, they now occur much less frequently.
Standardizing trauma care has also helped enhance patient care a great deal. Most major trauma centers have a “massive transfusion” protocol which allows the blood bank to prepare coolers containing not only blood, but also plasma, platelets, and procoagulants. This practice obviates the need to order the components individually. Rapid access to technology such as TEG allows emergency physicians (EPs) and other trauma care professionals to precisely guide transfusion therapy, but this remains an area of intensely debated investigation. Hopefully, our understanding will continue to mature over the next few years.
Another area of trauma care that has rapidly evolved is the use of endovascular techniques for trauma hemostasis. The realization that we can obtain control of vascular injury without the need for a large open operation has revolutionized care. While endovascular techniques have been used for pelvic hemostasis since 1972, we now use it regularly in every body cavity. Splenic artery embolization was developed by our (TS) group in Brooklyn, New York in 1995, and its use has now expanded to other abdominal solid organ injuries.
Injuries to the thoracic aorta once required a thoracotomy, cardiopulmonary bypass, and open repair. Stent grafting is now the treatment of choice for these injuries, allowing for a minimally invasive solution, and permitting those with both aortic and many other injuries to receive care for all of these wounds much sooner than was possible in the past, when multiply injured patients were simply not considered candidates for early open repair.
Thoracotomy in the ED has been widely practiced for a variety of indications. While it is still the only available solution for injury to the heart and/or proximal pulmonary vasculature in a patient who is hemodynamically unstable and/or in extremis, other options now exist to obtain vascular inflow for patients bleeding in the abdomen or pelvis. The use of transfemoral balloons for aortic occlusion allows clinicians to temporize hemorrhage without a huge open operation, and resuscitative endovascular balloon occlusion of the aorta (REBOA), has only been available for the last several years. The exact indications, wisest strategy, length of time the balloon can be inflated, rate of complications, and who is the appropriate physician (eg, EP, intensivist, vascular surgeon) to insert it, all remain questions requiring resolution. Much more work is necessary to pursue the role that REBOA can have in the care of desperately injured trauma patients.
There has been a revolution in the care of severely injured patients. After 50 years of thinking that we knew the answers, we have come to realize that those answers were wrong. Newer resuscitation strategies, as well as new treatment strategies continue to evolve, allowing us to refine care of severely injured patients. Perhaps the one thing we have really learned is that we do not have all of the answers and that the discussion must continue if we are to do better at serving more trauma victims.
Damage Control Resuscitation
In the United States, trauma is the leading cause of death in patients younger than age 45 years and ranks as the fifth leading cause of death among all age groups. Hemorrhage remains the leading cause of preventable death in the trauma population,1 and one of the most important recent changes in our care of the injured patient is the manner in which we manage hemorrhage. As noted earlier, there has been a paradigm shift away from large-volume crystalloid resuscitation and toward what has been termed “damage control resuscitation” (DCR).2,3
The principles of the DCR strategy are aimed at preemptively treating the lethal triad of hypothermia, acidosis, and coagulopathy in conjunction with control of surgical bleeding using damage control surgery. The main principles of DCR include “permissive hypotension,” prevention of heat loss and/or active warming, minimizing the use of crystalloid infusions, and initiating resuscitation with blood products in a ratio that more closely resembles whole blood.2
Permissive Hypotension
Permissive hypotension, also referred to as hypotensive resuscitation, is not considered a goal or an endpoint, but rather a “bridge” to definitive surgical control of hemorrhage. The body’s initial response to injury involves vasoconstriction and early clot formation, a process facilitated by hypotension. The rationale for permissive hypotension is that attempting to drive the BP up to normal ranges may interfere with vasoconstriction, as well as physically disrupting this early clot, leading to increased bleeding and further hypotension.
This concept has been corroborated by many animal and human studies.3 In 1994, the landmark study by Bickell et al4 randomized patients with penetrating torso trauma and a systolic BP (SBP) of 90 mm Hg or lower to either immediate or delayed fluid resuscitation. Their study demonstrated that patients whose fluid resuscitation was delayed until they reached the operating room had improved outcomes. The study supported the long-time prehospital practice of the “scoop-and-run” strategy, especially in penetrating torso trauma.
In 2003, Sondeen et al5 used a swine model of aortic injury to find an inflection point for clot disruption and re-bleeding during volume resuscitation. They found the inflection point to be a mean arterial pressure (MAP) of 64 mm Hg and an SBP of 94 mm Hg, regardless of the size of the aortotomy. Using an animal model of hemorrhagic shock, Li et al6 demonstrated in 2011 that resuscitation to a MAP of 50 mm Hg was associated with a decreased amount of blood loss as well as with improved survival compared to patients who were resuscitated to a MAP of 80 mm Hg. However, they also showed that after a time period of more than 90 to 120 minutes, the lower MAP group had increased end organ damage and worse outcomes, emphasizing the importance of prompt surgical control of bleeding—regardless of preoperative resuscitation strategy.
Other studies, though, have not shown a clear benefit to permissive hypotension. A 2002 study by Dutton et al7 showed that titration of initial fluid to a lower SBP (70 mm Hg) did not affect mortality when compared to a target resuscitation MAP of more than 100 mm Hg. Further, in 2014, a plenary paper presented to the American Association for the Surgery of Trauma demonstrated that controlled resuscitation (CR) strategy was safe and feasible,8 but did not demonstrate a mortality benefit in the overall cohort, though patients with blunt trauma who received CR had improved survival at 24 hours.
The group at Ben Taub General Hospital in Houston, Texas recently performed a randomized controlled trial evaluating intraoperative hypotensive resuscitation strategies. Patients in hemorrhagic shock were randomized to either an intraoperative MAP goal of 50 mm Hg or 65 mm Hg.9,10 Preliminary results suggested that targeting a lower MAP resulted in fewer blood product transfusions, less fluid administration, less coagulopathy, and lower mortality in the early postoperative period. Additionally, they demonstrated a nonsignificant trend toward improved 30-day mortality in the lower MAP group.9 Moreover, in this study there was no increased morbidity associated with the hypotensive strategy,10 suggesting that the approach was safe. Unfortunately, the trial was stopped early due to slow enrollment.
Despite the overall promising results with permissive hypotension, it is important to remember that it is contraindicated in patients with known or suspected traumatic brain injury, as hypotension has been shown to be detrimental in this population.11
Hemostatic Resuscitation and Coagulopathy
Avoiding Aggressive Crystalloid Resuscitation. While the ideal MAP to target during DCR remains unclear, the potential harm caused by aggressive crystalloid resuscitation has become more evident. Infusing excessive amounts of crystalloid has been shown to be associated with increased ventilator days, multisystem organ failure, abdominal compartment syndrome, and surgical-site infections12—all of which have also been associated with systemic consequences of increased inflammation, including increased release of tumor necrosis factor-alpha and other proinflammatory cytokines.13
Rodent studies have demonstrated large-volume crystalloid administration and breakdown or “thinning” of the endothelial glycocalyx, which leads to increased capillary leak, third-spacing, and ultimately intravascular volume depletion.14,15 This mechanism has been linked to pulmonary complications, namely acute lung injury and acute respiratory distress syndrome. Enteric edema resulting from aggressive crystalloid resuscitation has also been associated with prolonged postoperative ileus, increased risk of anastomotic leak,13 and inability to achieve primary fascial closure.16 All of the aforementioned complications are reduced when employing a restrictive fluid resuscitation strategy.17
Aggressive crystalloid administration in hemorrhagic shock also leads to dilutional coagulopathy. Multiple animal and human studies have shown an association between increased crystalloid volumes in hemorrhaging patients and increasing coagulopathy, blood loss, and mortality. In 2004, Barak et al18 demonstrated that administration of a high volume of crystalloid fluid (>3 L) or colloid (500 mL) was associated with postoperative coagulopathy; whereas in 2017, Harada et al,19 at Cedars-Sinai Medical Center in New York, demonstrated over a 10-year period that decreased high-volume (>2 L) crystalloid resuscitation paralleled a decrease in mortality.
Massive Transfusion Protocols. Many trauma centers have shifted away from high-volume crystalloid resuscitation in favor of massive transfusion protocols (MTPs) utilizing standardized ratios that more closely mimic whole blood. The MTPs center on the principle of equal transfusion ratios of blood product as opposed to packed RBCs (PRBCs) alone. This means effecting a plasma-rich resuscitation and preemptive correction of coagulopathy with FFP and platelets in addition to PRBCs.
Data from a US Army combat support hospital have demonstrated improved survival with an FFP to PRBC ratio of more than 1:1.4,20 and civilian studies have produced similar findings.21-23 All of these studies also noted improved mortality with higher (>1:2) platelet to PRBC ratios.22,23 Although, the ideal ratio remains unknown, many MTPs aim for 1:1:1 ratio (6 U FFP to 6 packed platelets to 6 U PRBC), which most closely mimics whole blood.
The Pragmatic Randomized Optimal Platelet and Plasma Ratios trial was a recent large multicenter randomized trial that compared transfusion ratios of 1:1:1 and 1:1:2. The trial was unable to demonstrate a difference in mortality at either 24 hours or 30 days, though more patients in the 1:1:1 ratio group achieved hemostasis and fewer patients in this group died from exsanguination in the first 24 hours.24Prehospital PRBC Administration. A number of studies have looked at prehospital administration of PRBCs.25-27 Holcomb et al25 showed no overall survival advantage at 24 hours, but did demonstrate a negligible blood-product wastage. In 2015 Brown et al26 found an increase probability of 24-hour survival, decreased shock, and lower 24-hour PRBC requirements with pretrauma-center PRBC transfusion. That same year Brown et al27 demonstrated that prehospital PRBC transfusion in severely injured blunt trauma patients was associated with decreased 24-hour and 30-day mortality rates, and a lower risk of coagulopathy. Currently, the Prehospital Air Medical Plasma trial is enrolling patients to evaluate the prehospital administration of plasma.28 The primary endpoint of the study is 30-day mortality; the tentative completion date for the study is January 2018.
Tranexamic Acid. Another important development in the treatment of hemorrhagic shock in recent years has been the use of TXA, an antifibrinolytic agent which inhibits the conversion of plasminogen to plasmin. It has been shown to decrease mortality in both civilian and military trauma populations.29,30
The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 trial was a large multicenter randomized trial, which showed a survival benefit among those who received TXA. The generalizability of the study has been questioned in the setting of modern urban trauma centers, as most of those enrolled in the study were from hospitals with no formal MTPs and a limited availability of blood products. Additionally, no laboratory measures of fibrinolysis were available.30
Most experts currently recommend TXA use as part of an MTP if there is evidence of hyperfibrinolysis on TEG or in severe hemorrhagic shock when the time from injury has been less than 3 hours, as studies have shown increased mortality when TXA was administered longer than 3 hours after injury.30
Viscoelastic Assays
An alternative approach to standardized ratio MTPs involves goal-directed hemostatic resuscitation using viscoelastic assays to guide transfusion of blood-product components. Both TEG and rotational thromboelastometry (ROTEM) are point-of-care tools for assessment of coagulation parameters of whole blood. Although they are not new technology, their use in trauma resuscitation is a relatively new concept.
While ROTEM is more commonly used in Europe, TEG is more popular and commonly used in the United States, though not exclusively.31,32
Thromboelastography
The TEG parameters most commonly used clinically are reaction time (R-time), kinetics time, angle, maximum amplitude (MA), and lysis at 30 minutes (LY30).
Reaction Time. The R-time is measured in minutes and represents the time to clot initiation, reflecting activity of coagulation factors. It is used in TEG-guided MTPs to trigger transfusion of FFP.31,32 The R-time is measured at the time the clot strength reaches an amplitude of 2 mm.31 The angle reflects the rate of rise of the amplitude of the TEG tracing, or the rate of increase in clot strength. Clinically, the angle represents fibrinogen concentration and function, and is used to trigger transfusion of cryoprecipitate or fibrinogen concentrate in MTPs.31,32
Maximum Amplitude. The MA is reached by the TEG tracing, or the maximum clot strength achieved. Although the MA has been shown to correlate with platelet count, it actually represents platelet count and function as well as fibrinogen activity, all of which contribute to clot strength. Clinically, MA is used to trigger platelet transfusion and/or administration of desmopressin in MTPs.31,32
Lysis at 30 Minutes. The LY30 is defined as the percent reduction in clot strength 30 minutes after reaching MA.31,32 Normal LY30 values are between 0% and 7.5%; however, these values have been challenged in recent studies, which have reported that an LY30 greater than 3% (termed hyperfibrinolysis) confers a significant increase in mortality and an increased likelihood of requiring massive transfusion.31,33 These findings have led to incorporation of this lower threshold as a trigger for administration of TXA during MTPs. Furthermore, an LY30 of less than 0.8% (described as fibrinolysis shutdown) has also been found to confer an increase in mortality,34 which has led many to advocate for goal-directed administration of TXA, rather than empiric administration, as these patients are more likely to be harmed than helped by such an intervention.31
Rapid Thromboelastography
Rapid TEG employs tissue factor to accelerate clot initiation and reaction time, providing an additional parameter which reflects coagulation factor activity: the activated clotting time (ACT).32
Activated Clotting Time. Historically used in cardiac surgery to measure anticoagulation during a cardiopulmonary bypass, ACT represents the same phase of coagulation as R-time, but is measured in seconds instead of minutes.31 The ACT has been found to correlate with prothrombin time/international normalized ratio (PT/INR), and accurately predicts the need for MTP.
Cotton et al35 found that an ACT of more than 128 seconds predicted patients requiring MTP, and an ACT lower than 105 seconds predicted those who required no transfusions in the first 24 hours after injury.35
The ACT can be used to trigger transfusion of FFP, but at certain thresholds, may also be used to trigger the early transfusion of cryoprecipitate and platelets.36 Moore et al36 found that an ACT over 140 seconds was able to predict an abnormal angle and MA. This had led to using this threshold as a trigger for early administration of cryoprecipitate and platelets, given this parameter is available within 5 minutes—long before the angle and MA have resulted.
Efficacy
The use of a TEG-guided strategy for MTP in trauma has shown great promise. In 2013, Tapia et al37 compared a historical cohort who received 1:1:1 MTP to a TEG-guided MTP and demonstrated improved mortality.In 2016, Gonzalez et al38 compared TEG-guided transfusion vs conventional coagulation tests (PT/INR, PTT, fibrinogen, platelets). The authors found a significant decrease in mortality and platelet and FFP transfusion when TEG-guided resuscitation is used.
Endovascular Techniques
The use of endovascular techniques in trauma continues to evolve. According to the National Trauma Data Bank, the use of endovascular therapies has increased from 1% of trauma cases in 2002 to 11% in 2008.39
Thoracic Endovascular Aortic Repair
Thoracic endovascular aortic repair (TEVAR) for blunt thoracic aortic injury has essentially replaced open surgical repair. (See Figures 1a and 1b for an example of a blunt traumatic aortic injury prior to and post-TEVAR placement.)
Transarterial Catheter Embolization
Endovascular treatments have also been used successfully in the management of injuries to aortic branch vessels and extremity vessels.42 Transarterial catheter embolization with coils, plugs, or gel foam is being employed with increasing frequency to achieve hemostasis in the pelvis and spleen.42 It may also be used as an adjunct to laparotomy and perihepatic packing in high-grade liver injuries, though it is associated with significant morbidity related to hepatic necrosis, bile leaks, and abscesses.43,44
Resuscitative Endovascular Balloon Occlusion of the Aorta
Most recently, REBOA has been used for noncompressible torso hemorrhage following trauma. This method involves percutaneous arterial cannulation of the common femoral artery and advancement of a balloon into the aorta, where it is then inflated at the desired level.
Once inflated, the balloon obstructs arterial inflow to the area of hemorrhage, curtailing blood loss, and increases proximal BP, improving coronary and cerebral perfusion. Multiple case reports and case series have described successful use of REBOA for hemorrhage control, including prehospital use by physicians in the United Kingdom. The largest series to date looked at 114 patients, of whom 46 had REBOA placement and 68 had open aortic occlusion through resuscitative thoracotomy.45 Those treated with REBOA were significantly more likely to achieve hemodynamic stability (defined as SBP >90 mm Hg for >5 minutes). Furthermore, the authors noted minimal complications from REBOA and no difference in time to successful aortic occlusion, regardless of technique. There was also no difference in mortality between the two groups. Despite the small number of studies in trauma patients, REBOA has been established as a viable alternative to open aortic occlusion. The prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery registry established by the American Association for the Surgery of Trauma is continuing to enroll patients and will hopefully answer many of the current uncertainties regarding the use of REBOA.
Conclusion
Strategies and techniques for the care of the injured patient have changed significantly in the past few years. Damage control resuscitation includes three elements: damage control surgery, permissive hypotension, and blood-product resuscitation.
The goals of lowering MAP in hemorrhagic shock appear to be safe and make sense physiologically, but have yet to show clear mortality benefit. Avoidance of excessive crystalloid resuscitation and trends toward more physiological ratios of blood product resuscitation have shown better outcomes. While the ideal ratio of blood products in transfusion remains unknown, the use of a massive transfusion strategy is preferable to crystalloid fluids. The use of viscoelastic assays (TEG and ROTEM) have allowed for goal-directed blood product resuscitation and may improve outcomes when compared with prescribed resuscitation ratios.
Finally, endovascular techniques in trauma have been increasingly utilized over the past 15 years, making nonoperative management with angiographic embolization for solid organ injury common practice now in most trauma centers worldwide. Temporary aortic balloon occlusion with REBOA appears promising in many cases of noncompressible truncal hemorrhage until definitive hemostasis can be achieved, but studies are needed to determine its ultimate place in the care of the trauma patient.
Introduction
For decades, virtually all injury was treated with open operative surgery. Resuscitation was based on the belief that large-volume crystalloid infusion to raise blood pressure (BP) to normal was the optimal therapy. Advanced trauma life support teaching was that 2 L of crystalloid fluid should be the initial resuscitation for all trauma patients, and those who failed to respond should receive additional crystalloid fluid. Patients did not receive a blood transfusion until later in treatment, and fresh frozen plasma (FFP) and platelets were not given until 10 U of blood had been administered. Regardless of the fluid infused, the goal of initial resuscitation was to raise BP to a normal level. During the time I (TS) was chair of the emergency medicine department at the State University of New York’s Kings County Hospital, I remember administering liters of crystalloid fluid preoperatively, believing it was not safe to operate until the patient had been what we termed “adequately resuscitated.”
However, as early as 1918, Walter B. Cannon, MD, correctly observed that fluid therapy without hemostasis was not wise, and numerous animal studies since then also raised serious questions about this approach. This article points out the revolutionary changes in the thinking and practice of resuscitation that have occurred in the last 20 years. We now realize that raising BP to normal only perpetuates hemorrhage. Hypotension treated with additional volume resuscitation without surgical control of hemorrhage creates a cycle leading to dilution of clotting factors and red blood cells (RBCs), recurrent hypotension, and ultimately death.
The realization that early blood transfusion is probably the wisest course is a concept that has only been in clinical practice for less than 15 years. Major trauma centers now routinely keep type O negative blood in the ED refrigerators so that it is instantly available.
Our understanding of trauma coagulopathy also has changed dramatically. Once thought to be simply a consequence of hypotension and hypothermia, we now realize that coagulopathy following trauma is far more complicated and likely occurs in concert with the inflammatory response to serious injury. Regardless of its etiology, we have recognized that earlier administration of plasma and platelets following trauma prevents coagulopathy, and this approach is more beneficial than treating coagulopathy after it develops. There has been much debate about the optimal ratios of RBCs, plasma, and platelets, and the ideal ratio has yet to be determined. The idea that “one-size-fits-all” is almost certainly not the case: Different patients require different and more precise treatment strategies.
For years, we have relied on laboratory measurements of coagulation to guide transfusion therapy, but standard laboratory values often take over 30 minutes to obtain. In an extremely dynamic situation involving large-volume blood loss, this interval is too lax. A more personalized approach using rapidly available technology, such as thromboelastography (TEG), allows for real-time assessment of a multiplicity of coagulation dynamics and rapid correction of any abnormalities. Procoagulants such as factor 7A, prothrombin complex concentrate (PCC), and tranexamic acid (TXA) have a role. However, the data to support the use of these expensive agents is lacking. While they certainly can be life-saving, each of these components brings with it a risk of causing indiscriminate coagulation—even in areas of the body that are not injured. Moreover, their availability in nontrauma centers is either limited or not an option.
There is little question that our rapid advances in understanding resuscitation and transfusion practice has saved lives. Twenty years ago, intensive care units were populated by trauma patients who had received many liters of crystalloid fluid, and at least partly a consequence of the resuscitation experience, many had severe respiratory failure. Open abdomens were common and also a likely consequence of large-volume crystalloid use. While these problems have not entirely disappeared, they now occur much less frequently.
Standardizing trauma care has also helped enhance patient care a great deal. Most major trauma centers have a “massive transfusion” protocol which allows the blood bank to prepare coolers containing not only blood, but also plasma, platelets, and procoagulants. This practice obviates the need to order the components individually. Rapid access to technology such as TEG allows emergency physicians (EPs) and other trauma care professionals to precisely guide transfusion therapy, but this remains an area of intensely debated investigation. Hopefully, our understanding will continue to mature over the next few years.
Another area of trauma care that has rapidly evolved is the use of endovascular techniques for trauma hemostasis. The realization that we can obtain control of vascular injury without the need for a large open operation has revolutionized care. While endovascular techniques have been used for pelvic hemostasis since 1972, we now use it regularly in every body cavity. Splenic artery embolization was developed by our (TS) group in Brooklyn, New York in 1995, and its use has now expanded to other abdominal solid organ injuries.
Injuries to the thoracic aorta once required a thoracotomy, cardiopulmonary bypass, and open repair. Stent grafting is now the treatment of choice for these injuries, allowing for a minimally invasive solution, and permitting those with both aortic and many other injuries to receive care for all of these wounds much sooner than was possible in the past, when multiply injured patients were simply not considered candidates for early open repair.
Thoracotomy in the ED has been widely practiced for a variety of indications. While it is still the only available solution for injury to the heart and/or proximal pulmonary vasculature in a patient who is hemodynamically unstable and/or in extremis, other options now exist to obtain vascular inflow for patients bleeding in the abdomen or pelvis. The use of transfemoral balloons for aortic occlusion allows clinicians to temporize hemorrhage without a huge open operation, and resuscitative endovascular balloon occlusion of the aorta (REBOA), has only been available for the last several years. The exact indications, wisest strategy, length of time the balloon can be inflated, rate of complications, and who is the appropriate physician (eg, EP, intensivist, vascular surgeon) to insert it, all remain questions requiring resolution. Much more work is necessary to pursue the role that REBOA can have in the care of desperately injured trauma patients.
There has been a revolution in the care of severely injured patients. After 50 years of thinking that we knew the answers, we have come to realize that those answers were wrong. Newer resuscitation strategies, as well as new treatment strategies continue to evolve, allowing us to refine care of severely injured patients. Perhaps the one thing we have really learned is that we do not have all of the answers and that the discussion must continue if we are to do better at serving more trauma victims.
Damage Control Resuscitation
In the United States, trauma is the leading cause of death in patients younger than age 45 years and ranks as the fifth leading cause of death among all age groups. Hemorrhage remains the leading cause of preventable death in the trauma population,1 and one of the most important recent changes in our care of the injured patient is the manner in which we manage hemorrhage. As noted earlier, there has been a paradigm shift away from large-volume crystalloid resuscitation and toward what has been termed “damage control resuscitation” (DCR).2,3
The principles of the DCR strategy are aimed at preemptively treating the lethal triad of hypothermia, acidosis, and coagulopathy in conjunction with control of surgical bleeding using damage control surgery. The main principles of DCR include “permissive hypotension,” prevention of heat loss and/or active warming, minimizing the use of crystalloid infusions, and initiating resuscitation with blood products in a ratio that more closely resembles whole blood.2
Permissive Hypotension
Permissive hypotension, also referred to as hypotensive resuscitation, is not considered a goal or an endpoint, but rather a “bridge” to definitive surgical control of hemorrhage. The body’s initial response to injury involves vasoconstriction and early clot formation, a process facilitated by hypotension. The rationale for permissive hypotension is that attempting to drive the BP up to normal ranges may interfere with vasoconstriction, as well as physically disrupting this early clot, leading to increased bleeding and further hypotension.
This concept has been corroborated by many animal and human studies.3 In 1994, the landmark study by Bickell et al4 randomized patients with penetrating torso trauma and a systolic BP (SBP) of 90 mm Hg or lower to either immediate or delayed fluid resuscitation. Their study demonstrated that patients whose fluid resuscitation was delayed until they reached the operating room had improved outcomes. The study supported the long-time prehospital practice of the “scoop-and-run” strategy, especially in penetrating torso trauma.
In 2003, Sondeen et al5 used a swine model of aortic injury to find an inflection point for clot disruption and re-bleeding during volume resuscitation. They found the inflection point to be a mean arterial pressure (MAP) of 64 mm Hg and an SBP of 94 mm Hg, regardless of the size of the aortotomy. Using an animal model of hemorrhagic shock, Li et al6 demonstrated in 2011 that resuscitation to a MAP of 50 mm Hg was associated with a decreased amount of blood loss as well as with improved survival compared to patients who were resuscitated to a MAP of 80 mm Hg. However, they also showed that after a time period of more than 90 to 120 minutes, the lower MAP group had increased end organ damage and worse outcomes, emphasizing the importance of prompt surgical control of bleeding—regardless of preoperative resuscitation strategy.
Other studies, though, have not shown a clear benefit to permissive hypotension. A 2002 study by Dutton et al7 showed that titration of initial fluid to a lower SBP (70 mm Hg) did not affect mortality when compared to a target resuscitation MAP of more than 100 mm Hg. Further, in 2014, a plenary paper presented to the American Association for the Surgery of Trauma demonstrated that controlled resuscitation (CR) strategy was safe and feasible,8 but did not demonstrate a mortality benefit in the overall cohort, though patients with blunt trauma who received CR had improved survival at 24 hours.
The group at Ben Taub General Hospital in Houston, Texas recently performed a randomized controlled trial evaluating intraoperative hypotensive resuscitation strategies. Patients in hemorrhagic shock were randomized to either an intraoperative MAP goal of 50 mm Hg or 65 mm Hg.9,10 Preliminary results suggested that targeting a lower MAP resulted in fewer blood product transfusions, less fluid administration, less coagulopathy, and lower mortality in the early postoperative period. Additionally, they demonstrated a nonsignificant trend toward improved 30-day mortality in the lower MAP group.9 Moreover, in this study there was no increased morbidity associated with the hypotensive strategy,10 suggesting that the approach was safe. Unfortunately, the trial was stopped early due to slow enrollment.
Despite the overall promising results with permissive hypotension, it is important to remember that it is contraindicated in patients with known or suspected traumatic brain injury, as hypotension has been shown to be detrimental in this population.11
Hemostatic Resuscitation and Coagulopathy
Avoiding Aggressive Crystalloid Resuscitation. While the ideal MAP to target during DCR remains unclear, the potential harm caused by aggressive crystalloid resuscitation has become more evident. Infusing excessive amounts of crystalloid has been shown to be associated with increased ventilator days, multisystem organ failure, abdominal compartment syndrome, and surgical-site infections12—all of which have also been associated with systemic consequences of increased inflammation, including increased release of tumor necrosis factor-alpha and other proinflammatory cytokines.13
Rodent studies have demonstrated large-volume crystalloid administration and breakdown or “thinning” of the endothelial glycocalyx, which leads to increased capillary leak, third-spacing, and ultimately intravascular volume depletion.14,15 This mechanism has been linked to pulmonary complications, namely acute lung injury and acute respiratory distress syndrome. Enteric edema resulting from aggressive crystalloid resuscitation has also been associated with prolonged postoperative ileus, increased risk of anastomotic leak,13 and inability to achieve primary fascial closure.16 All of the aforementioned complications are reduced when employing a restrictive fluid resuscitation strategy.17
Aggressive crystalloid administration in hemorrhagic shock also leads to dilutional coagulopathy. Multiple animal and human studies have shown an association between increased crystalloid volumes in hemorrhaging patients and increasing coagulopathy, blood loss, and mortality. In 2004, Barak et al18 demonstrated that administration of a high volume of crystalloid fluid (>3 L) or colloid (500 mL) was associated with postoperative coagulopathy; whereas in 2017, Harada et al,19 at Cedars-Sinai Medical Center in New York, demonstrated over a 10-year period that decreased high-volume (>2 L) crystalloid resuscitation paralleled a decrease in mortality.
Massive Transfusion Protocols. Many trauma centers have shifted away from high-volume crystalloid resuscitation in favor of massive transfusion protocols (MTPs) utilizing standardized ratios that more closely mimic whole blood. The MTPs center on the principle of equal transfusion ratios of blood product as opposed to packed RBCs (PRBCs) alone. This means effecting a plasma-rich resuscitation and preemptive correction of coagulopathy with FFP and platelets in addition to PRBCs.
Data from a US Army combat support hospital have demonstrated improved survival with an FFP to PRBC ratio of more than 1:1.4,20 and civilian studies have produced similar findings.21-23 All of these studies also noted improved mortality with higher (>1:2) platelet to PRBC ratios.22,23 Although, the ideal ratio remains unknown, many MTPs aim for 1:1:1 ratio (6 U FFP to 6 packed platelets to 6 U PRBC), which most closely mimics whole blood.
The Pragmatic Randomized Optimal Platelet and Plasma Ratios trial was a recent large multicenter randomized trial that compared transfusion ratios of 1:1:1 and 1:1:2. The trial was unable to demonstrate a difference in mortality at either 24 hours or 30 days, though more patients in the 1:1:1 ratio group achieved hemostasis and fewer patients in this group died from exsanguination in the first 24 hours.24Prehospital PRBC Administration. A number of studies have looked at prehospital administration of PRBCs.25-27 Holcomb et al25 showed no overall survival advantage at 24 hours, but did demonstrate a negligible blood-product wastage. In 2015 Brown et al26 found an increase probability of 24-hour survival, decreased shock, and lower 24-hour PRBC requirements with pretrauma-center PRBC transfusion. That same year Brown et al27 demonstrated that prehospital PRBC transfusion in severely injured blunt trauma patients was associated with decreased 24-hour and 30-day mortality rates, and a lower risk of coagulopathy. Currently, the Prehospital Air Medical Plasma trial is enrolling patients to evaluate the prehospital administration of plasma.28 The primary endpoint of the study is 30-day mortality; the tentative completion date for the study is January 2018.
Tranexamic Acid. Another important development in the treatment of hemorrhagic shock in recent years has been the use of TXA, an antifibrinolytic agent which inhibits the conversion of plasminogen to plasmin. It has been shown to decrease mortality in both civilian and military trauma populations.29,30
The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 trial was a large multicenter randomized trial, which showed a survival benefit among those who received TXA. The generalizability of the study has been questioned in the setting of modern urban trauma centers, as most of those enrolled in the study were from hospitals with no formal MTPs and a limited availability of blood products. Additionally, no laboratory measures of fibrinolysis were available.30
Most experts currently recommend TXA use as part of an MTP if there is evidence of hyperfibrinolysis on TEG or in severe hemorrhagic shock when the time from injury has been less than 3 hours, as studies have shown increased mortality when TXA was administered longer than 3 hours after injury.30
Viscoelastic Assays
An alternative approach to standardized ratio MTPs involves goal-directed hemostatic resuscitation using viscoelastic assays to guide transfusion of blood-product components. Both TEG and rotational thromboelastometry (ROTEM) are point-of-care tools for assessment of coagulation parameters of whole blood. Although they are not new technology, their use in trauma resuscitation is a relatively new concept.
While ROTEM is more commonly used in Europe, TEG is more popular and commonly used in the United States, though not exclusively.31,32
Thromboelastography
The TEG parameters most commonly used clinically are reaction time (R-time), kinetics time, angle, maximum amplitude (MA), and lysis at 30 minutes (LY30).
Reaction Time. The R-time is measured in minutes and represents the time to clot initiation, reflecting activity of coagulation factors. It is used in TEG-guided MTPs to trigger transfusion of FFP.31,32 The R-time is measured at the time the clot strength reaches an amplitude of 2 mm.31 The angle reflects the rate of rise of the amplitude of the TEG tracing, or the rate of increase in clot strength. Clinically, the angle represents fibrinogen concentration and function, and is used to trigger transfusion of cryoprecipitate or fibrinogen concentrate in MTPs.31,32
Maximum Amplitude. The MA is reached by the TEG tracing, or the maximum clot strength achieved. Although the MA has been shown to correlate with platelet count, it actually represents platelet count and function as well as fibrinogen activity, all of which contribute to clot strength. Clinically, MA is used to trigger platelet transfusion and/or administration of desmopressin in MTPs.31,32
Lysis at 30 Minutes. The LY30 is defined as the percent reduction in clot strength 30 minutes after reaching MA.31,32 Normal LY30 values are between 0% and 7.5%; however, these values have been challenged in recent studies, which have reported that an LY30 greater than 3% (termed hyperfibrinolysis) confers a significant increase in mortality and an increased likelihood of requiring massive transfusion.31,33 These findings have led to incorporation of this lower threshold as a trigger for administration of TXA during MTPs. Furthermore, an LY30 of less than 0.8% (described as fibrinolysis shutdown) has also been found to confer an increase in mortality,34 which has led many to advocate for goal-directed administration of TXA, rather than empiric administration, as these patients are more likely to be harmed than helped by such an intervention.31
Rapid Thromboelastography
Rapid TEG employs tissue factor to accelerate clot initiation and reaction time, providing an additional parameter which reflects coagulation factor activity: the activated clotting time (ACT).32
Activated Clotting Time. Historically used in cardiac surgery to measure anticoagulation during a cardiopulmonary bypass, ACT represents the same phase of coagulation as R-time, but is measured in seconds instead of minutes.31 The ACT has been found to correlate with prothrombin time/international normalized ratio (PT/INR), and accurately predicts the need for MTP.
Cotton et al35 found that an ACT of more than 128 seconds predicted patients requiring MTP, and an ACT lower than 105 seconds predicted those who required no transfusions in the first 24 hours after injury.35
The ACT can be used to trigger transfusion of FFP, but at certain thresholds, may also be used to trigger the early transfusion of cryoprecipitate and platelets.36 Moore et al36 found that an ACT over 140 seconds was able to predict an abnormal angle and MA. This had led to using this threshold as a trigger for early administration of cryoprecipitate and platelets, given this parameter is available within 5 minutes—long before the angle and MA have resulted.
Efficacy
The use of a TEG-guided strategy for MTP in trauma has shown great promise. In 2013, Tapia et al37 compared a historical cohort who received 1:1:1 MTP to a TEG-guided MTP and demonstrated improved mortality.In 2016, Gonzalez et al38 compared TEG-guided transfusion vs conventional coagulation tests (PT/INR, PTT, fibrinogen, platelets). The authors found a significant decrease in mortality and platelet and FFP transfusion when TEG-guided resuscitation is used.
Endovascular Techniques
The use of endovascular techniques in trauma continues to evolve. According to the National Trauma Data Bank, the use of endovascular therapies has increased from 1% of trauma cases in 2002 to 11% in 2008.39
Thoracic Endovascular Aortic Repair
Thoracic endovascular aortic repair (TEVAR) for blunt thoracic aortic injury has essentially replaced open surgical repair. (See Figures 1a and 1b for an example of a blunt traumatic aortic injury prior to and post-TEVAR placement.)
Transarterial Catheter Embolization
Endovascular treatments have also been used successfully in the management of injuries to aortic branch vessels and extremity vessels.42 Transarterial catheter embolization with coils, plugs, or gel foam is being employed with increasing frequency to achieve hemostasis in the pelvis and spleen.42 It may also be used as an adjunct to laparotomy and perihepatic packing in high-grade liver injuries, though it is associated with significant morbidity related to hepatic necrosis, bile leaks, and abscesses.43,44
Resuscitative Endovascular Balloon Occlusion of the Aorta
Most recently, REBOA has been used for noncompressible torso hemorrhage following trauma. This method involves percutaneous arterial cannulation of the common femoral artery and advancement of a balloon into the aorta, where it is then inflated at the desired level.
Once inflated, the balloon obstructs arterial inflow to the area of hemorrhage, curtailing blood loss, and increases proximal BP, improving coronary and cerebral perfusion. Multiple case reports and case series have described successful use of REBOA for hemorrhage control, including prehospital use by physicians in the United Kingdom. The largest series to date looked at 114 patients, of whom 46 had REBOA placement and 68 had open aortic occlusion through resuscitative thoracotomy.45 Those treated with REBOA were significantly more likely to achieve hemodynamic stability (defined as SBP >90 mm Hg for >5 minutes). Furthermore, the authors noted minimal complications from REBOA and no difference in time to successful aortic occlusion, regardless of technique. There was also no difference in mortality between the two groups. Despite the small number of studies in trauma patients, REBOA has been established as a viable alternative to open aortic occlusion. The prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery registry established by the American Association for the Surgery of Trauma is continuing to enroll patients and will hopefully answer many of the current uncertainties regarding the use of REBOA.
Conclusion
Strategies and techniques for the care of the injured patient have changed significantly in the past few years. Damage control resuscitation includes three elements: damage control surgery, permissive hypotension, and blood-product resuscitation.
The goals of lowering MAP in hemorrhagic shock appear to be safe and make sense physiologically, but have yet to show clear mortality benefit. Avoidance of excessive crystalloid resuscitation and trends toward more physiological ratios of blood product resuscitation have shown better outcomes. While the ideal ratio of blood products in transfusion remains unknown, the use of a massive transfusion strategy is preferable to crystalloid fluids. The use of viscoelastic assays (TEG and ROTEM) have allowed for goal-directed blood product resuscitation and may improve outcomes when compared with prescribed resuscitation ratios.
Finally, endovascular techniques in trauma have been increasingly utilized over the past 15 years, making nonoperative management with angiographic embolization for solid organ injury common practice now in most trauma centers worldwide. Temporary aortic balloon occlusion with REBOA appears promising in many cases of noncompressible truncal hemorrhage until definitive hemostasis can be achieved, but studies are needed to determine its ultimate place in the care of the trauma patient.
1. Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158-163. doi:10.1007/s00268-009-0266-1.
2. Bogert JN, Harvin JA, Cotton BA. Damage control resuscitation. J Intensive Care Med. 2016;31(3):177-186. doi:10.1177/0885066614558018.
3. Kaafarani HMA, Velmahos GC. Damage control resuscitation in trauma. Scand J Surg. 2014;103(2):81-88. doi:10.1177/1457496914524388.
4. Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105-1109. doi:10.1056/NEJM199410273311701.
5. Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110-S117. doi:10.1097/01.TA.0000047220.81795.3D.
6. Li T, Zhu Y, Hu Y, et al. Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology. 2011;114(1):111-119. doi:10.1097/ALN.0b013e3181fe3fe7.
7. Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52(6):1141-1146.
8. Schreiber MA, Meier EN, Tisherman SA, et al; ROC Investigators. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687-695. doi:10.1097/TA.0000000000000600.
9. Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652-663. doi:10.1097/TA.0b013e31820e77ea.
10. Carrick MM, Morrison CA, Tapia NM, et al. Intraoperative hypotensive resuscitation for patients undergoing laparotomy or thoracotomy for trauma: Early termination of a randomized prospective clinical trial. J Trauma Acute Care Surg. 2016;80(6):886-896. doi:10.1097/TA.0000000000001044.
11. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216-222.
12. Kasotakis G, Sideris A, Yang Y, et al. Inflammation and Host Response to Injury Investigators. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74(5):1215-1221; discussion 1221-1222. doi:10.1097/TA.0b013e3182826e13.
13. Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115-121. doi:10.1097/01.shk.0000209564.84822.f2.
14. Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289-1295. doi:10.1213/ANE.0b013e318210385c.
15. Torres LN, Sondeen JL, Ji L, Dubick MA, Filho IT. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759-766. doi:10.1097/TA.0b013e3182a92514.
16. Bradley M, Galvagno S, Dhanda A, et al. Damage control resuscitation protocol and the management of open abdomens in trauma patients. Am Surg. 2014;80(8):768-775.
17. Brandstrup B, Tønnesen H, Beier-Holgersen R, et al; Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641-648. doi:10.1097/01.sla.0000094387.50865.23.
18. Barak M, Rudin M, Vofsi O, Droyan A, Katz Y. Fluid administration during abdominal surgery influences on coagulation in the postoperative period. Curr Surg. 2004;61(5):459-462. doi:10.1016/j.cursur.2004.02.002.
19. Harada MY, Ko A, Barmparas G, et al. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int J Surg. 2017;38:78-82. doi:10.1016/j.ijsu.2016.12.073.
20. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805-813. doi:10.1097/TA.0b013e3181271ba3.
21. Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177-1782; discussion 1182-1183. doi:10.1097/TA.0b013e31816c5c80.
22. Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447-458. doi:10.1097/SLA.0b013e318185a9ad.
23. Holcomb JB, del Junco DJ, Fox EE, et al; PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127-136. doi:10.1001/2013.jamasurg.387.
24. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
25. Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care. 2015;19(1):1-9. doi:10.3109/10903127.2014.923077.
26. Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg. 2015;220(5):797-808. doi:10.1016/j.jamcollsurg.2015.01.006.
27. Brown JB, Cohen MJ, Minei JP, et al; Inflammation and the Host Response to Injury Investigators. Pretrauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured patients with blunt trauma. Ann Surg. 2015;261(5):997-1005. doi:10.1097/SLA.0000000000000674.
28. Brown JB, Guyette FX, Neal MD, et al. Taking the blood bank to the field: the design and rationale of the Prehospital Air Medical Plasma (PAMPer) trial. Prehosp Emerg Care. 2015;19(3):343-350. doi:10.3109/10903127.2014.995851.
29. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119. doi:10.1001/archsurg.2011.287.
30. Shakur H, Roberts I, Bautista R, et al; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi:10.1016/S0140-6736(10)60835-5.
31. Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119-134. doi:10.1016/j.ccc.2016.09.002.
32. Abdelfattah K, Cripps MW. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg. 2016;33(Pt B):196-201. doi:10.1016/j.ijsu.2015.09.036.
33. Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365-370; discussion 370. doi:10.1097/TA.0b013e31825c1234.
34. Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811-817. doi:10.1097/TA.0000000000000341.
35. Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71(2):407-414; discussion 414-417. doi:10.1097/TA.0b013e31821e1bf0.
36. Moore HB, Moore EE, Chin TL, et al. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014;156(3):564-569. doi:10.1016/j.surg.2014.04.017.
37. Tapia NM, Chang A, Norman M, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378-385; discussion 385-386. doi:10.1097/TA.0b013e31827e20e0.
38. Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051-1059. doi:10.1097/SLA.0000000000001608.
39. Avery LE, Stahlfeld KR, Corcos AC, et al. Evolving role of endovascular techniques for traumatic vascular injury: a changing landscape? J Trauma Acute Care Surg. 2012;72(1):41-46; discussion 46-47. doi:10.1097/TA.0b013e31823d0f03.
40. Demetriades D, Velmahos GC, Scalea TM, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma. 2008;64(6):1415-1418; discussion 1418-1419. doi:10.1097/TA.0b013e3181715e32.
41. Azizzadeh A, Ray HM, Dubose JJ, et al. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg. 2014;76(2):510-516. doi:10.1097/TA.0b013e3182aafe8c.
42. Brenner M, Hoehn M, Rasmussen TE. Endovascular therapy in trauma. Eur J Trauma Emerg Surg. 2014;40(6):671-678. doi:10.1007/s00068-014-0474-8.
43. Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma. 2009;66(3):621-627; discussion 627-629. doi:10.1097/TA.0b013e31819919f2.
44. Letoublon C, Morra I, Chen Y, Monnin V, Voirin D, Arvieux C. Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma. 2011;70(5):1032-1036; discussion 1036-1037. doi:10.1097/TA.0b013e31820e7ca1.
45. DuBose JJ, Scalea TM, Brenner M, et al; AORTA Study Group. The AAST prospective Aortic Occlusion for Resuscitati on in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419. doi:10.1097/TA.0000000000001079.
1. Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158-163. doi:10.1007/s00268-009-0266-1.
2. Bogert JN, Harvin JA, Cotton BA. Damage control resuscitation. J Intensive Care Med. 2016;31(3):177-186. doi:10.1177/0885066614558018.
3. Kaafarani HMA, Velmahos GC. Damage control resuscitation in trauma. Scand J Surg. 2014;103(2):81-88. doi:10.1177/1457496914524388.
4. Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105-1109. doi:10.1056/NEJM199410273311701.
5. Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110-S117. doi:10.1097/01.TA.0000047220.81795.3D.
6. Li T, Zhu Y, Hu Y, et al. Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology. 2011;114(1):111-119. doi:10.1097/ALN.0b013e3181fe3fe7.
7. Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52(6):1141-1146.
8. Schreiber MA, Meier EN, Tisherman SA, et al; ROC Investigators. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687-695. doi:10.1097/TA.0000000000000600.
9. Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652-663. doi:10.1097/TA.0b013e31820e77ea.
10. Carrick MM, Morrison CA, Tapia NM, et al. Intraoperative hypotensive resuscitation for patients undergoing laparotomy or thoracotomy for trauma: Early termination of a randomized prospective clinical trial. J Trauma Acute Care Surg. 2016;80(6):886-896. doi:10.1097/TA.0000000000001044.
11. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216-222.
12. Kasotakis G, Sideris A, Yang Y, et al. Inflammation and Host Response to Injury Investigators. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74(5):1215-1221; discussion 1221-1222. doi:10.1097/TA.0b013e3182826e13.
13. Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115-121. doi:10.1097/01.shk.0000209564.84822.f2.
14. Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289-1295. doi:10.1213/ANE.0b013e318210385c.
15. Torres LN, Sondeen JL, Ji L, Dubick MA, Filho IT. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759-766. doi:10.1097/TA.0b013e3182a92514.
16. Bradley M, Galvagno S, Dhanda A, et al. Damage control resuscitation protocol and the management of open abdomens in trauma patients. Am Surg. 2014;80(8):768-775.
17. Brandstrup B, Tønnesen H, Beier-Holgersen R, et al; Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641-648. doi:10.1097/01.sla.0000094387.50865.23.
18. Barak M, Rudin M, Vofsi O, Droyan A, Katz Y. Fluid administration during abdominal surgery influences on coagulation in the postoperative period. Curr Surg. 2004;61(5):459-462. doi:10.1016/j.cursur.2004.02.002.
19. Harada MY, Ko A, Barmparas G, et al. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int J Surg. 2017;38:78-82. doi:10.1016/j.ijsu.2016.12.073.
20. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805-813. doi:10.1097/TA.0b013e3181271ba3.
21. Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177-1782; discussion 1182-1183. doi:10.1097/TA.0b013e31816c5c80.
22. Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447-458. doi:10.1097/SLA.0b013e318185a9ad.
23. Holcomb JB, del Junco DJ, Fox EE, et al; PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127-136. doi:10.1001/2013.jamasurg.387.
24. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
25. Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care. 2015;19(1):1-9. doi:10.3109/10903127.2014.923077.
26. Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg. 2015;220(5):797-808. doi:10.1016/j.jamcollsurg.2015.01.006.
27. Brown JB, Cohen MJ, Minei JP, et al; Inflammation and the Host Response to Injury Investigators. Pretrauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured patients with blunt trauma. Ann Surg. 2015;261(5):997-1005. doi:10.1097/SLA.0000000000000674.
28. Brown JB, Guyette FX, Neal MD, et al. Taking the blood bank to the field: the design and rationale of the Prehospital Air Medical Plasma (PAMPer) trial. Prehosp Emerg Care. 2015;19(3):343-350. doi:10.3109/10903127.2014.995851.
29. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119. doi:10.1001/archsurg.2011.287.
30. Shakur H, Roberts I, Bautista R, et al; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi:10.1016/S0140-6736(10)60835-5.
31. Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119-134. doi:10.1016/j.ccc.2016.09.002.
32. Abdelfattah K, Cripps MW. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg. 2016;33(Pt B):196-201. doi:10.1016/j.ijsu.2015.09.036.
33. Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365-370; discussion 370. doi:10.1097/TA.0b013e31825c1234.
34. Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811-817. doi:10.1097/TA.0000000000000341.
35. Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71(2):407-414; discussion 414-417. doi:10.1097/TA.0b013e31821e1bf0.
36. Moore HB, Moore EE, Chin TL, et al. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014;156(3):564-569. doi:10.1016/j.surg.2014.04.017.
37. Tapia NM, Chang A, Norman M, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378-385; discussion 385-386. doi:10.1097/TA.0b013e31827e20e0.
38. Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051-1059. doi:10.1097/SLA.0000000000001608.
39. Avery LE, Stahlfeld KR, Corcos AC, et al. Evolving role of endovascular techniques for traumatic vascular injury: a changing landscape? J Trauma Acute Care Surg. 2012;72(1):41-46; discussion 46-47. doi:10.1097/TA.0b013e31823d0f03.
40. Demetriades D, Velmahos GC, Scalea TM, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma. 2008;64(6):1415-1418; discussion 1418-1419. doi:10.1097/TA.0b013e3181715e32.
41. Azizzadeh A, Ray HM, Dubose JJ, et al. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg. 2014;76(2):510-516. doi:10.1097/TA.0b013e3182aafe8c.
42. Brenner M, Hoehn M, Rasmussen TE. Endovascular therapy in trauma. Eur J Trauma Emerg Surg. 2014;40(6):671-678. doi:10.1007/s00068-014-0474-8.
43. Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma. 2009;66(3):621-627; discussion 627-629. doi:10.1097/TA.0b013e31819919f2.
44. Letoublon C, Morra I, Chen Y, Monnin V, Voirin D, Arvieux C. Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications. J Trauma. 2011;70(5):1032-1036; discussion 1036-1037. doi:10.1097/TA.0b013e31820e7ca1.
45. DuBose JJ, Scalea TM, Brenner M, et al; AORTA Study Group. The AAST prospective Aortic Occlusion for Resuscitati on in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419. doi:10.1097/TA.0000000000001079.