User login
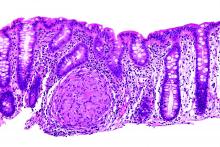
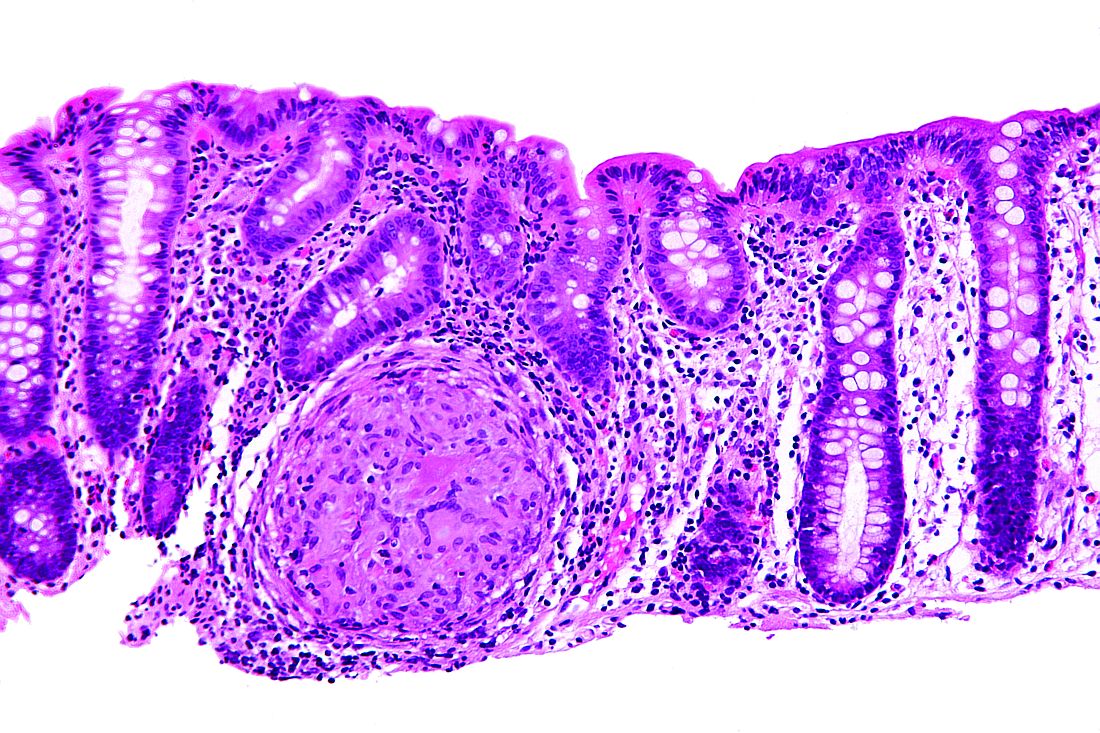
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
FROM ANALES DE PEDIATRIA