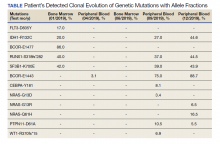
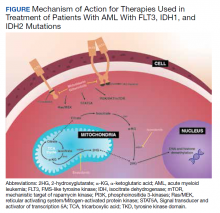
User login
Migraine: Helping patients through a difficult journey
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
Most clinicians who treat migraine know the statistics associated with this debilitating condition and can recite them almost verbatim. Likewise, we all know the long journey a patient experiencing headache can take before finding relief.
The question is, what can we do about it? It can sometimes feel easy to look at the statistics, accept them with little objection, and move on, but we must do the best we can for our patients in the face of the migraine challenge.
It’s a journey that I welcome you to take with me as we evaluate important migraine trends, treatments, and controversies, and figure out how to leverage these developments to improve outcomes and help our patients improve and feel and function better.
There are upwards of 39 million migraine sufferers in the US who are on a journey of their own that is often perplexing, frustrating, and can feel fruitless. According to the Migraine Research Foundation, most migraine sufferers do not seek medical treatment. More than half of patients who experience migraine are never diagnosed. Moreover, only 4% receive care from headache and pain specialists. Perhaps most disheartening, an estimated 5 million migraine sufferers, who we believe can benefit from preventive treatment, are not receiving it.
The patient journey can be extremely discouraging and often maddening. Results from the American Migraine Study show that nearly four in every 10 migraine patients suffer 3 years or more before being diagnosed. A cross-sectional study published in 2019 analyzed treatments, procedures, and follow-up approaches experienced by 456 migraine sufferers until their initial consult with a headache specialist. Patients reported an average headache frequency of approximately 16 days per month. More than half were found to have chronic migraine, and 3 in every 10 had migraine with aura. Despite these characteristics—which were apparently hiding in plain sight—it took patients in this study an average of about 17 years from pain onset to make the journey to an appointment with a headache specialist. That is hard to believe, let alone to understand. Along the way, many migraineurs—particularly those with chronic migraine—were subjected to unnecessary exams and treatments.
Results like these do not come cheaply for families, society, and the health care system. Migraine is estimated to cost more than $20 million per year in direct medical expenses and lost productivity in the US, according to the American Migraine Foundation. Others have estimated double that number. Sufferers, meanwhile, face the prospect of significant pain, stigma and ongoing disability. More than 8 in every 10participants in the American Migraine Study had at least some headache-related disability. More than half say their pain caused severe impairment, even requiring bed rest.
I believe we can help our migraine patients along this journey—and we can make it less arduous for them. We have education, tools, and treatments to help them. Learn how by joining me here each month. We will address the practical relevance of topics such as acute and preventive care (including the new gepants and CGRP-targeted treatments), new and more effective treatments for medication overuse headache, new treatment devices, behavioral approaches to migraine, and our role in headache advocacy, including stigma avoidance. We will not shy away from controversial topics such as the changing definition of chronic migraine, monoclonal antibody safety , high and low cerebrospinal fluid pressure syndromes, and more. See you next month.
Concern over response to COVID-19 in patients with blood cancers
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bedside EEG test aids prognosis in patients with brain injury
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Physicians react: Doctors worry about patients reading their clinical notes
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Ultrasound ablation for Parkinson’s disease: Benefit limited by adverse effects
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Sequential Targeted Treatment for a Geriatric Patient with Acute Myeloid Leukemia with Concurrent FLT3-TKD and IDH1 Mutations
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Which imaging criteria identify progressive forms of MS?
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
The role of imaging in diagnosing progressive multiple sclerosis (MS) and in assessing prognosis is the subject of a new review.
MRI is central in the diagnostic work-up of patients suspected of having MS, given its high sensitivity in detecting disease dissemination in space and over time and its notable ability to exclude mimics of MS, the authors noted. However, diagnosis of primary progressive MS remains challenging and is only possible retrospectively on the basis of clinical assessment.
they wrote.
Diagnosis of progressive MS is limited by difficulties in distinguishing accumulating disability caused by inflammatory disease activity from that attributable to degenerative processes associated with secondary progressive MS. Moreover, there are no accepted clinical criteria for diagnosing secondary progressive MS, the authors explained.
This need has promoted extensive research in the field of imaging, facilitated by definition of novel MRI sequences, to identify imaging features reflecting pathophysiological mechanisms relevant to the pathobiology of progressive MS, the authors said.
The current review reports the conclusions of a workshop held in Milan in November 2019, at which an expert panel of neurologists and neuroradiologists addressed the role of MRI in progressive MS.
Massimo Filippi, MD, IRCCS San Raffaele Scientific Institute, Milan, was the lead author of the review, which was published online Dec. 14, 2020, in JAMA Neurology.
The authors concluded that no definitive, qualitative clinical, immunologic, histopathologic, or neuroimaging features differentiate primary progressive and secondary progressive forms of MS; both are characterized by neurodegenerative phenomena and a gradual and irreversible accumulation of clinical disability, which is also affected by aging and comorbidities.
A definitive diagnosis of primary progressive MS is more difficult than a diagnosis of relapsing remitting MS; in part, primary progressive MS is a diagnosis of exclusion because it can be mimicked by other conditions clinically and radiologically, the authors noted.
The writers did report that, although nonspecific, some spinal cord imaging features are typical of primary progressive MS. These include diffuse abnormalities and lesions involving gray matter and two or more white-matter columns, but confirmation of this is required.
In patients with primary progressive MS and those with relapse-onset MS, MRI features at disease onset predict long-term disability and a progressive disease course. These features include lesions in critical central nervous system regions (i.e., spinal cord, infratentorial regions, and gray matter) and high inflammatory activity in the first years after disease onset. These measures are evaluable in clinical practice, the authors said.
In patients with established MS, gray-matter involvement and neurodegeneration are associated with accelerated clinical worsening; however, detection validation and standardization need to be implemented at the individual patient level, they commented.
Novel candidate imaging biomarkers, such as subpial demyelination, and the presence of slowly expanding lesions or paramagnetic rim lesions may identify progressive MS but should be further investigated, they added.
Discovery of MRI markers capable of detecting evolution from relapsing-remitting to secondary progressive MS remains an unmet need that will probably require multiparametric MRI studies, because it is unlikely that a single MRI method will be able to allow clinicians to optimally distinguish among these stages, the authors said.
The contribution of these promising MRI measures combined with other biomarkers, such as quantification of serum neurofilament light chain levels or optical coherence tomography assessment, should be explored to improve the identification of patients with progressive MS, they concluded.
‘A comprehensive review’
In a comment, Jeffrey A. Cohen, MD, director of the Cleveland Clinic’s Mellen Center for MS Treatment and Research, said the article is a comprehensive review of the pathologic mechanisms that underlie progression in MS and the proxy measures of those processes (brain and spinal cord MRI, PET, optical coherence tomography, and biomarkers).
“The paper reports there is no qualitative difference between relapsing remitting and progressive MS; rather, the difference is quantitative,” Dr. Cohen noted. “In other words, the processes that underlie progression are present from the earliest stages of MS, becoming more prominent over time.”
The apparent transition to progressive MS, he added, “rather than representing a ‘transition,’ instead results from the accumulation of pathology over time, a shift from focal lesions to diffuse inflammation and damage, and unmasking of the damage due to decreased resiliency due to aging and failure of compensatory mechanisms (neuroplasticity and remyelination).”
Also commenting, Edward Fox, MD, director, MS Clinic of Central Texas and clinical associate professor, University of Texas, Austin, explained that loss of tissue is the main driver of progressive MS.
“We all look at imaging to confirm that the progressive symptoms expressed by the patient are related to demyelinating disease,” he said. “When I see MRI of the spinal cord showing multifocal lesions, especially if localized atrophy is seen in a region of the cord, I expect to hear a history of progressive deficits in gait and other signs of disability.”
Dr. Fox noted that, on MRI of the brain, gray matter atrophy both cortically and in the deep gray structures usually manifests as cognitive slowing and poorer performance in work and social situations.
“We hope that other biomarkers, such as neurofilament light chain, will add to this body of knowledge and give us a better grasp of the definition of neurodegeneration to confirm the clinical and radiographic findings,” he added.
Dr. Filippi has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi, Genzyme, Takeda, and Teva Pharmaceutical Industries; and research support from ARiSLA, Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, Roche, and Teva.
A version of this article first appeared on Medscape.com.
New evidence shows that COVID-19 invades the brain
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Experts offer roadmap for treating CLL during the pandemic
COVID-19 has thrown a wrench in standard treatment protocols for patients with chronic lymphocytic leukemia (CLL). These patients already face a greater risk of dying from infections, and recent research suggests they tend to have risk factors that increase their likelihood of complications and death from COVID-19.
In August, a group of oncologists from the United States and Europe published a literature-informed expert opinion to help their colleagues navigate this new CLL treatment landscape. It offers a roadmap for balancing patients’ therapeutic needs against their risk for viral infection and outlines the safest course of action for patients who test positive for COVID-19.
Mazyar Shadman, MD, MPH, an associate professor in the Clinical Research Division of the Fred Hutchinson Cancer Research Center and the Division of Medical Oncology at the University of Washington School of Medicine, in Seattle, Washington, was contacted for comment to break down what clinicians need to know about treating CLL during the pandemic. This interview has been edited for length and clarity.
Question: What prompted you and colleagues from the United States and Europe to write these recommendations?
Dr. Shadman: When we began the collaboration earlier this year, our colleagues in Italy and the rest of Europe had more experience with COVID-19, so they led the effort. We wanted to help oncologists manage their patients with CLL during the pandemic based on the evidence we had at the time and the unknowns we faced.
What’s an example of how the available evidence informed your recommendations?
At the time, we didn’t know whether patients with CLL were more likely to get COVID-19, compared to the general population, but we did have evidence already that cancer increases patients’ risk of bad outcomes and death from COVID-19. CLL, for example, can increase risk factors for infection, including hypogammaglobulinemia, innate immune dysfunction, and neutropenia, which may be exacerbated by anticancer treatments. Patients’ existing immune suppression might prevent or delay their ability to react to or cope with the virus. And many patients with CLL have other conditions that increase their risk of a severe response to COVID-19, including older age (70% of CLL patients are older than 65 years), hypertension (21%), and diabetes (26%).
These factors informed our recommendations to limit patients’ exposure to COVID-19 by reducing or postponing the number of in-person visits and routine in-hospital follow-ups, especially if they could be substituted with virtual check-ins.
The expert opinion recommendations are divided into three main categories: patients who are newly diagnosed with CLL but have not begun receiving therapy, those already receiving therapy but are free of COVID-19, and those who test positive for COVID-19. Let’s start with the first category. What do the recommendations say about waiting versus proceeding for newly diagnosed patients?
Our priority was balancing the negative impacts of getting COVID-19 with the negative impacts of postponing cancer treatment. We suggested taking each new CLL case on a patient-by-patient basis to determine who needed treatment tomorrow and who could wait a few weeks or months. Fortunately, CLL rarely requires immediate therapy, so the preference was to postpone treatment a few weeks, depending on the local COVID-19 outbreak situation.
In my practice, for instance, we tried to postpone visits as much as we could. Before the pandemic, patients with CLL in the watch-and-wait phase – those diagnosed but who don’t require treatment immediately – would come in for bloodwork and exams every 3-6 months. But when the pandemic hit, we skipped 3-month visits for patients with stable lab results and switched to telehealth visits instead. For those who needed blood draws, we used local labs closer to the patient’s home to minimize their exposure and transportation requirements.
When treatment cannot be deferred, we’ve recommended starting patients on therapies that require fewer in-person visits and are less immune suppressive. We recommended oncologists consider Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib and acalabrutinib, as well as venetoclax. Some research suggests these inhibitors may be protective against COVID-19 by blunting a patient’s hyperinflammatory response to the virus. These drugs also require minimal routine treatment and lab visits, which helps limit patients’ potential exposure to COVID-19.
But there are risks to waiting. Even during the peak of the pandemic here in Seattle, if patients needed treatment immediately, we did not delay. Patients with significant drops in their platelet or neutrophil count or those with bulky disease, for instance, do require therapy.
It’s important to mention that we did have bad experiences with patients who needed immediate treatment and their treating physicians decided to wait because of COVID-19 risks. These patients who came in with aggressive CLL and experienced delays in care had much more complicated CLL treatment than if they had started treatment earlier.
When organ function became abnormal, for example, some patients could no longer receive certain therapies. If someone’s kidney function becomes abnormal, I wouldn’t recommend giving a drug like venetoclax. Although rare, some patients on venetoclax develop tumor lysis syndrome, which can lead to kidney failure.
Bottom line: Don’t just assume it’s a low-grade disease and that you can wait.
What about patients already receiving treatment for CLL who are free of COVID-19?
For patients on active treatment, we suggested stopping or holding treatment with monoclonal antibodies, such as rituximab and obinutuzumab, and chemotherapy regimens, such as idelalisib plus rituximab and duvelisib, when possible. We recommended oncologists consider continuing treatment for patients on BTK inhibitors.
What happens if a patient with CLL tests positive for COVID-19?
If a patient tests positive for COVID-19 but is not yet on CLL treatment, we recommend postponing CLL care until they’ve recovered from the infection. If a patient is already receiving treatment, the recommendations are similar to those above for COVID-19–negative patients: Delay care for those on chemotherapy and monoclonal antibodies, but consider continuing treatment for patients on BTK inhibitors.
The expert opinion was submitted in May and ultimately published in August. How has our understanding of treating CLL during the pandemic changed since then? Would you change any recommendations?
When we published this paper, it was still early on in the pandemic, and we didn’t know as much about COVID-19 and CLL as we do now. Since we published the recommendations, we have received confirmation from several studies that patients with cancer have a more complicated course of COVID-19 and have worse outcomes. But I believe the recommendations we devised early in the pandemic still hold now. Decisions about delivering treatment should be influenced by the local COVID-19 numbers and hospital resources as well as the patient’s specific situation – whether they have more stable disease and can delay or postpone care or whether they need more immediate attention.
With a further surge in cases predicted as we move even deeper into flu season, what would you recommend for initiating treatment in newly diagnosed patients?
The pandemic has created a very fluid situation for treating CLL. What’s happening now in Seattle may not be the same story in New York, California, or elsewhere. In early November [when Dr. Shadman was first contacted], in Seattle, we were not postponing care because our COVID-19 numbers were fairly good. But, as of mid December, that is starting to change as the COVID-19 numbers fluctuate.
If we do experience a second peak of COVID-19 cases, we would need to modify our practice as we did during the initial surge earlier this year. That would mean avoiding treatment with monoclonal antibodies and chemotherapy, as well as minimizing blood draws and drugs that require frequent in-person visits.
How important is it for patients to be vaccinated against COVID-19?
There are two key things to consider about a vaccine. Is the vaccine safe from the general safety standpoint that everyone is worried about? And if the vaccine is not harmful, will it work in patients will CLL?
Because we don’t yet know the complete side-effect profile of a COVID-19 vaccine, we would need to assess each patient’s condition to limit adverse reactions and to see whether the vaccine alters a patient’s immune response to the CLL drug they’re taking.
At the University of Washington, Seattle, we have a plan to start studying the effectiveness of the Pfizer and Moderna vaccines in patients with CLL – carefully assessing patients’ response to the vaccine in terms of antibody response. We already know, based on small studies, that the antibody response to the flu vaccine, for instance, is not as strong in patients with CLL, compared to those without. But, overall, as long as the vaccine won’t cause harm, I would recommend my patients get it.
Dr. Shadman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 has thrown a wrench in standard treatment protocols for patients with chronic lymphocytic leukemia (CLL). These patients already face a greater risk of dying from infections, and recent research suggests they tend to have risk factors that increase their likelihood of complications and death from COVID-19.
In August, a group of oncologists from the United States and Europe published a literature-informed expert opinion to help their colleagues navigate this new CLL treatment landscape. It offers a roadmap for balancing patients’ therapeutic needs against their risk for viral infection and outlines the safest course of action for patients who test positive for COVID-19.
Mazyar Shadman, MD, MPH, an associate professor in the Clinical Research Division of the Fred Hutchinson Cancer Research Center and the Division of Medical Oncology at the University of Washington School of Medicine, in Seattle, Washington, was contacted for comment to break down what clinicians need to know about treating CLL during the pandemic. This interview has been edited for length and clarity.
Question: What prompted you and colleagues from the United States and Europe to write these recommendations?
Dr. Shadman: When we began the collaboration earlier this year, our colleagues in Italy and the rest of Europe had more experience with COVID-19, so they led the effort. We wanted to help oncologists manage their patients with CLL during the pandemic based on the evidence we had at the time and the unknowns we faced.
What’s an example of how the available evidence informed your recommendations?
At the time, we didn’t know whether patients with CLL were more likely to get COVID-19, compared to the general population, but we did have evidence already that cancer increases patients’ risk of bad outcomes and death from COVID-19. CLL, for example, can increase risk factors for infection, including hypogammaglobulinemia, innate immune dysfunction, and neutropenia, which may be exacerbated by anticancer treatments. Patients’ existing immune suppression might prevent or delay their ability to react to or cope with the virus. And many patients with CLL have other conditions that increase their risk of a severe response to COVID-19, including older age (70% of CLL patients are older than 65 years), hypertension (21%), and diabetes (26%).
These factors informed our recommendations to limit patients’ exposure to COVID-19 by reducing or postponing the number of in-person visits and routine in-hospital follow-ups, especially if they could be substituted with virtual check-ins.
The expert opinion recommendations are divided into three main categories: patients who are newly diagnosed with CLL but have not begun receiving therapy, those already receiving therapy but are free of COVID-19, and those who test positive for COVID-19. Let’s start with the first category. What do the recommendations say about waiting versus proceeding for newly diagnosed patients?
Our priority was balancing the negative impacts of getting COVID-19 with the negative impacts of postponing cancer treatment. We suggested taking each new CLL case on a patient-by-patient basis to determine who needed treatment tomorrow and who could wait a few weeks or months. Fortunately, CLL rarely requires immediate therapy, so the preference was to postpone treatment a few weeks, depending on the local COVID-19 outbreak situation.
In my practice, for instance, we tried to postpone visits as much as we could. Before the pandemic, patients with CLL in the watch-and-wait phase – those diagnosed but who don’t require treatment immediately – would come in for bloodwork and exams every 3-6 months. But when the pandemic hit, we skipped 3-month visits for patients with stable lab results and switched to telehealth visits instead. For those who needed blood draws, we used local labs closer to the patient’s home to minimize their exposure and transportation requirements.
When treatment cannot be deferred, we’ve recommended starting patients on therapies that require fewer in-person visits and are less immune suppressive. We recommended oncologists consider Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib and acalabrutinib, as well as venetoclax. Some research suggests these inhibitors may be protective against COVID-19 by blunting a patient’s hyperinflammatory response to the virus. These drugs also require minimal routine treatment and lab visits, which helps limit patients’ potential exposure to COVID-19.
But there are risks to waiting. Even during the peak of the pandemic here in Seattle, if patients needed treatment immediately, we did not delay. Patients with significant drops in their platelet or neutrophil count or those with bulky disease, for instance, do require therapy.
It’s important to mention that we did have bad experiences with patients who needed immediate treatment and their treating physicians decided to wait because of COVID-19 risks. These patients who came in with aggressive CLL and experienced delays in care had much more complicated CLL treatment than if they had started treatment earlier.
When organ function became abnormal, for example, some patients could no longer receive certain therapies. If someone’s kidney function becomes abnormal, I wouldn’t recommend giving a drug like venetoclax. Although rare, some patients on venetoclax develop tumor lysis syndrome, which can lead to kidney failure.
Bottom line: Don’t just assume it’s a low-grade disease and that you can wait.
What about patients already receiving treatment for CLL who are free of COVID-19?
For patients on active treatment, we suggested stopping or holding treatment with monoclonal antibodies, such as rituximab and obinutuzumab, and chemotherapy regimens, such as idelalisib plus rituximab and duvelisib, when possible. We recommended oncologists consider continuing treatment for patients on BTK inhibitors.
What happens if a patient with CLL tests positive for COVID-19?
If a patient tests positive for COVID-19 but is not yet on CLL treatment, we recommend postponing CLL care until they’ve recovered from the infection. If a patient is already receiving treatment, the recommendations are similar to those above for COVID-19–negative patients: Delay care for those on chemotherapy and monoclonal antibodies, but consider continuing treatment for patients on BTK inhibitors.
The expert opinion was submitted in May and ultimately published in August. How has our understanding of treating CLL during the pandemic changed since then? Would you change any recommendations?
When we published this paper, it was still early on in the pandemic, and we didn’t know as much about COVID-19 and CLL as we do now. Since we published the recommendations, we have received confirmation from several studies that patients with cancer have a more complicated course of COVID-19 and have worse outcomes. But I believe the recommendations we devised early in the pandemic still hold now. Decisions about delivering treatment should be influenced by the local COVID-19 numbers and hospital resources as well as the patient’s specific situation – whether they have more stable disease and can delay or postpone care or whether they need more immediate attention.
With a further surge in cases predicted as we move even deeper into flu season, what would you recommend for initiating treatment in newly diagnosed patients?
The pandemic has created a very fluid situation for treating CLL. What’s happening now in Seattle may not be the same story in New York, California, or elsewhere. In early November [when Dr. Shadman was first contacted], in Seattle, we were not postponing care because our COVID-19 numbers were fairly good. But, as of mid December, that is starting to change as the COVID-19 numbers fluctuate.
If we do experience a second peak of COVID-19 cases, we would need to modify our practice as we did during the initial surge earlier this year. That would mean avoiding treatment with monoclonal antibodies and chemotherapy, as well as minimizing blood draws and drugs that require frequent in-person visits.
How important is it for patients to be vaccinated against COVID-19?
There are two key things to consider about a vaccine. Is the vaccine safe from the general safety standpoint that everyone is worried about? And if the vaccine is not harmful, will it work in patients will CLL?
Because we don’t yet know the complete side-effect profile of a COVID-19 vaccine, we would need to assess each patient’s condition to limit adverse reactions and to see whether the vaccine alters a patient’s immune response to the CLL drug they’re taking.
At the University of Washington, Seattle, we have a plan to start studying the effectiveness of the Pfizer and Moderna vaccines in patients with CLL – carefully assessing patients’ response to the vaccine in terms of antibody response. We already know, based on small studies, that the antibody response to the flu vaccine, for instance, is not as strong in patients with CLL, compared to those without. But, overall, as long as the vaccine won’t cause harm, I would recommend my patients get it.
Dr. Shadman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 has thrown a wrench in standard treatment protocols for patients with chronic lymphocytic leukemia (CLL). These patients already face a greater risk of dying from infections, and recent research suggests they tend to have risk factors that increase their likelihood of complications and death from COVID-19.
In August, a group of oncologists from the United States and Europe published a literature-informed expert opinion to help their colleagues navigate this new CLL treatment landscape. It offers a roadmap for balancing patients’ therapeutic needs against their risk for viral infection and outlines the safest course of action for patients who test positive for COVID-19.
Mazyar Shadman, MD, MPH, an associate professor in the Clinical Research Division of the Fred Hutchinson Cancer Research Center and the Division of Medical Oncology at the University of Washington School of Medicine, in Seattle, Washington, was contacted for comment to break down what clinicians need to know about treating CLL during the pandemic. This interview has been edited for length and clarity.
Question: What prompted you and colleagues from the United States and Europe to write these recommendations?
Dr. Shadman: When we began the collaboration earlier this year, our colleagues in Italy and the rest of Europe had more experience with COVID-19, so they led the effort. We wanted to help oncologists manage their patients with CLL during the pandemic based on the evidence we had at the time and the unknowns we faced.
What’s an example of how the available evidence informed your recommendations?
At the time, we didn’t know whether patients with CLL were more likely to get COVID-19, compared to the general population, but we did have evidence already that cancer increases patients’ risk of bad outcomes and death from COVID-19. CLL, for example, can increase risk factors for infection, including hypogammaglobulinemia, innate immune dysfunction, and neutropenia, which may be exacerbated by anticancer treatments. Patients’ existing immune suppression might prevent or delay their ability to react to or cope with the virus. And many patients with CLL have other conditions that increase their risk of a severe response to COVID-19, including older age (70% of CLL patients are older than 65 years), hypertension (21%), and diabetes (26%).
These factors informed our recommendations to limit patients’ exposure to COVID-19 by reducing or postponing the number of in-person visits and routine in-hospital follow-ups, especially if they could be substituted with virtual check-ins.
The expert opinion recommendations are divided into three main categories: patients who are newly diagnosed with CLL but have not begun receiving therapy, those already receiving therapy but are free of COVID-19, and those who test positive for COVID-19. Let’s start with the first category. What do the recommendations say about waiting versus proceeding for newly diagnosed patients?
Our priority was balancing the negative impacts of getting COVID-19 with the negative impacts of postponing cancer treatment. We suggested taking each new CLL case on a patient-by-patient basis to determine who needed treatment tomorrow and who could wait a few weeks or months. Fortunately, CLL rarely requires immediate therapy, so the preference was to postpone treatment a few weeks, depending on the local COVID-19 outbreak situation.
In my practice, for instance, we tried to postpone visits as much as we could. Before the pandemic, patients with CLL in the watch-and-wait phase – those diagnosed but who don’t require treatment immediately – would come in for bloodwork and exams every 3-6 months. But when the pandemic hit, we skipped 3-month visits for patients with stable lab results and switched to telehealth visits instead. For those who needed blood draws, we used local labs closer to the patient’s home to minimize their exposure and transportation requirements.
When treatment cannot be deferred, we’ve recommended starting patients on therapies that require fewer in-person visits and are less immune suppressive. We recommended oncologists consider Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib and acalabrutinib, as well as venetoclax. Some research suggests these inhibitors may be protective against COVID-19 by blunting a patient’s hyperinflammatory response to the virus. These drugs also require minimal routine treatment and lab visits, which helps limit patients’ potential exposure to COVID-19.
But there are risks to waiting. Even during the peak of the pandemic here in Seattle, if patients needed treatment immediately, we did not delay. Patients with significant drops in their platelet or neutrophil count or those with bulky disease, for instance, do require therapy.
It’s important to mention that we did have bad experiences with patients who needed immediate treatment and their treating physicians decided to wait because of COVID-19 risks. These patients who came in with aggressive CLL and experienced delays in care had much more complicated CLL treatment than if they had started treatment earlier.
When organ function became abnormal, for example, some patients could no longer receive certain therapies. If someone’s kidney function becomes abnormal, I wouldn’t recommend giving a drug like venetoclax. Although rare, some patients on venetoclax develop tumor lysis syndrome, which can lead to kidney failure.
Bottom line: Don’t just assume it’s a low-grade disease and that you can wait.
What about patients already receiving treatment for CLL who are free of COVID-19?
For patients on active treatment, we suggested stopping or holding treatment with monoclonal antibodies, such as rituximab and obinutuzumab, and chemotherapy regimens, such as idelalisib plus rituximab and duvelisib, when possible. We recommended oncologists consider continuing treatment for patients on BTK inhibitors.
What happens if a patient with CLL tests positive for COVID-19?
If a patient tests positive for COVID-19 but is not yet on CLL treatment, we recommend postponing CLL care until they’ve recovered from the infection. If a patient is already receiving treatment, the recommendations are similar to those above for COVID-19–negative patients: Delay care for those on chemotherapy and monoclonal antibodies, but consider continuing treatment for patients on BTK inhibitors.
The expert opinion was submitted in May and ultimately published in August. How has our understanding of treating CLL during the pandemic changed since then? Would you change any recommendations?
When we published this paper, it was still early on in the pandemic, and we didn’t know as much about COVID-19 and CLL as we do now. Since we published the recommendations, we have received confirmation from several studies that patients with cancer have a more complicated course of COVID-19 and have worse outcomes. But I believe the recommendations we devised early in the pandemic still hold now. Decisions about delivering treatment should be influenced by the local COVID-19 numbers and hospital resources as well as the patient’s specific situation – whether they have more stable disease and can delay or postpone care or whether they need more immediate attention.
With a further surge in cases predicted as we move even deeper into flu season, what would you recommend for initiating treatment in newly diagnosed patients?
The pandemic has created a very fluid situation for treating CLL. What’s happening now in Seattle may not be the same story in New York, California, or elsewhere. In early November [when Dr. Shadman was first contacted], in Seattle, we were not postponing care because our COVID-19 numbers were fairly good. But, as of mid December, that is starting to change as the COVID-19 numbers fluctuate.
If we do experience a second peak of COVID-19 cases, we would need to modify our practice as we did during the initial surge earlier this year. That would mean avoiding treatment with monoclonal antibodies and chemotherapy, as well as minimizing blood draws and drugs that require frequent in-person visits.
How important is it for patients to be vaccinated against COVID-19?
There are two key things to consider about a vaccine. Is the vaccine safe from the general safety standpoint that everyone is worried about? And if the vaccine is not harmful, will it work in patients will CLL?
Because we don’t yet know the complete side-effect profile of a COVID-19 vaccine, we would need to assess each patient’s condition to limit adverse reactions and to see whether the vaccine alters a patient’s immune response to the CLL drug they’re taking.
At the University of Washington, Seattle, we have a plan to start studying the effectiveness of the Pfizer and Moderna vaccines in patients with CLL – carefully assessing patients’ response to the vaccine in terms of antibody response. We already know, based on small studies, that the antibody response to the flu vaccine, for instance, is not as strong in patients with CLL, compared to those without. But, overall, as long as the vaccine won’t cause harm, I would recommend my patients get it.
Dr. Shadman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
High blood pressure at any age speeds cognitive decline
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 15,000 participants, hypertension during middle age was associated with memory decline, and onset at later ages was linked to worsening memory and global cognition.
The investigators found that prehypertension, defined as systolic pressure of 120-139 mm Hg or diastolic pressure of 80-89 mm Hg, was also linked to accelerated cognitive decline.
Although duration of hypertension was not associated with any marker of cognitive decline, blood pressure control “can substantially reduce hypertension’s deleterious effect on the pace of cognitive decline,” said study investigator Sandhi M. Barreto, MD, PhD, professor of medicine at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
The findings were published online Dec. 14 in Hypertension.
Unanswered questions
Hypertension is an established and highly prevalent risk factor for cognitive decline, but the age at which it begins to affect cognition is unclear. Previous research suggests that onset during middle age is associated with more harmful cognitive effects than onset in later life. One reason for this apparent difference may be that the duration of hypertension influences the magnitude of cognitive decline, the researchers noted.
Other studies have shown that prehypertension is associated with damage to certain organs, but its effects on cognition are uncertain. In addition, the effect of good blood pressure control with antihypertensive medications and the impact on cognition are also unclear.
To investigate, the researchers examined data from the ongoing, multicenter ELSA-Brasil study. ELSA-Brasil follows 15,105 civil servants between the ages of 35 and 74 years. Dr. Barreto and team assessed data from visit 1, which was conducted between 2008 and 2010, and visit 2, which was conducted between 2012 and 2014.
At each visit, participants underwent a memory test, a verbal fluency test, and the Trail Making Test Part B. The investigators calculated Z scores for these tests to derive a global cognitive score.
Blood pressure was measured on the right arm, and hypertension status, age at the time of hypertension diagnosis, duration of hypertension diagnosis, hypertension treatment, and control status were recorded. Other covariables included sex, education, race, smoking status, physical activity, body mass index, and total cholesterol level.
The researchers excluded patients who did not undergo cognitive testing at visit 2, those who had a history of stroke at baseline, and those who initiated antihypertensive medications despite having normotension. After exclusions, the analysis included 7,063 participants (approximately 55% were women, 15% were Black).
At visit 1, the mean age of the group was 58.9 years, and 53.4% of participants had 14 or more years of education. In addition, 22% had prehypertension, and 46.8% had hypertension. The median duration of hypertension was 7 years; 29.8% of participants with hypertension were diagnosed with the condition during middle age.
Of those who reported having hypertension at visit 1, 7.3% were not taking any antihypertensive medication. Among participants with hypertension who were taking antihypertensives, 31.2% had uncontrolled blood pressure.
Independent predictor
Results showed that prehypertension independently predicted a significantly greater decline in verbal fluency (Z score, –0.0095; P < .01) and global cognitive score (Z score, –0.0049; P < .05) compared with normal blood pressure.
At middle age, hypertension was associated with a steeper decline in memory (Z score, –0.0072; P < .05) compared with normal blood pressure. At older ages, hypertension was linked to a steeper decline in both memory (Z score, –0.0151; P < .001) and global cognitive score (Z score, –0.0080; P < .01). Duration of hypertension, however, did not significantly predict changes in cognition (P < .109).
Among those with hypertension who were taking antihypertensive medications, those with uncontrolled blood pressure experienced greater declines in rapid memory (Z score, –0.0126; P < .01) and global cognitive score (Z score, –0.0074; P < .01) than did those with controlled blood pressure.
The investigators noted that the study participants had a comparatively high level of education, which has been shown to “boost cognitive reserve and lessen the speed of age-related cognitive decline,” Dr. Barreto said. However, “our results indicate that the effect of hypertension on cognitive decline affects individuals of all educational levels similarly,” she said.
Dr. Barreto noted that the findings have two major clinical implications. First, “maintaining blood pressure below prehypertension levels is important to preserve cognitive function or delay cognitive decline,” she said. Secondly, “in hypertensive individuals, keeping blood pressure under control is essential to reduce the speed of cognitive decline.”
The researchers plan to conduct further analyses of the data to clarify the observed relationship between memory and verbal fluency. They also plan to examine how hypertension affects long-term executive function.
‘Continuum of risk’
Commenting on the study, Philip B. Gorelick, MD, MPH, adjunct professor of neurology (stroke and neurocritical care) at Northwestern University, Chicago, noted that, so far, research suggests that the risk for stroke associated with blood pressure levels should be understood as representing a continuum rather than as being associated with several discrete points.
“The same may hold true for cognitive decline and dementia. There may be a continuum of risk whereby persons even at so-called elevated but relatively lower levels of blood pressure based on a continuous scale are at risk,” said Dr. Gorelick, who was not involved with the current study.
The investigators relied on a large and well-studied population of civil servants. However, the population’s relative youth and high level of education may limit the generalizability of the findings, he noted. In addition, the follow-up time was relatively short.
“The hard endpoint of dementia was not studied but would be of interest to enhance our understanding of the influence of blood pressure elevation on cognitive decline or dementia during a longer follow-up of the cohort,” Dr. Gorelick said.
The findings also suggest the need to better understand mechanisms that link blood pressure elevation with cognitive decline, he added.
They indicate “the need for additional clinical trials to better elucidate blood pressure lowering targets for cognitive preservation in different groups of persons at risk,” such as those with normal cognition, those with mild cognitive impairment, and those with dementia, said Dr. Gorelick. “For example, is it safe and efficacious to lower blood pressure in persons with more advanced cognitive impairment or dementia?” he asked.
The study was funded by the Brazilian Coordination for the Improvement of Higher Education Personnel. Dr. Barreto has received support from the Research Agency of the State of Minas Gerais. Although Dr. Gorelick was not involved in the ELSA-Brasil cohort study, he serves on a data monitoring committee for a trial of a blood pressure–lowering agent in the preservation of cognition.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION