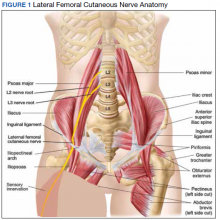
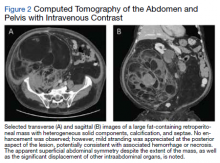
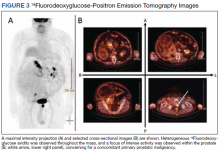
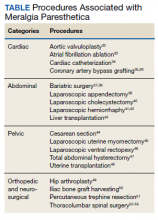
User login
Sustained long-term benefit of gene therapy for SMA
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
Medication overuse headache: Preventive treatment with or without detoxification?
The goal of treating medication overuse headache is obvious: ceasing overuse of the medication in question in an effort to return to a headache pattern that is episodic and better managed. Although guidelines suggest withdrawal of the overused medication and initiating preventive treatment, there is debate about this approach versus withdrawal alone or preventive treatment without ceasing the overused medication. A recently published randomized trial from Carlsen and colleagues evaluated 3 treatment methods: 1) withdrawal plus preventive treatment; 2) preventive treatment only; and 3) withdrawal followed by optional preventive treatment 2 months after withdrawal. Investigators found all 3 approaches effective, but participants who underwent withdrawal plus preventive care saw their headache days reduced by 12.3 days, versus 9.9 days in the preventive-only group and 8.5 days in the withdrawal/optional preventive follow-up treatment contingent. No statistically significant differences were seen between the groups in terms of migraine days, days with short-term medication use, and headache pain intensity.
Particularly noteworthy was the finding that individuals treated with withdrawal plus preventive treatment were significantly more likely to achieve remission. Specifically, nearly 75% returned to experiencing episodic headache, compared with 60% in the preventive group and 42% in the withdrawal contingent. Nearly all (97%) of those on the withdrawal plus preventive regimen were cured of medication overuse headache, versus 90% (withdrawal) and 74% (preventive).
The bottom line: Individuals undergoing withdrawal plus preventive treatment were 30% more likely to be cured of medication overuse headache. Thus, it appears that detoxification is key.
Or is it?
On the one hand…
In studies, withdrawal from the offending medication is linked with substantial improvement in headache days. Additionally, individuals who previously responded poorly to preventive treatment fared better with such treatment after detoxification.
When treating medication overuse headache using the detoxification and preventive care approach, Sun-Edelstein and colleagues outline these important steps:
- Educate your patients and their family/caregivers about the detoxification process
- Wean patient off the offending medication with a goal of complete detoxification
- Initiate preventive medical therapy or behavioral/non-drug strategies
- Establish clear limits on acute medication intake
- Arrange for regular follow-up to minimize or prevent relapse
While on the other hand…
Even though guidelines recommend detoxification, there is data supporting the concept of initiating preventive treatment without detoxification. A randomized, double-blind, placebo-controlled trial by Mei and colleagues found that 100 mg per day of topiramate led to a significant reduction in headache days and average amount of acute medication intake, versus placebo. However, treatment completion rates were low, leading Sun-Edelstein and colleagues to surmise that topiramate without detoxification would probably not have had a high success rate in practice.
Meanwhile, onabotulinumtoxin A was found in the PREEMPT trials conducted by Dodick and colleagues to reduce the number of headache days, migraine days, and moderate/severe headache days, compared with placebo, at week 24. Disappointingly, researchers found that acute medication frequency was not reduced in the overall treatment group, but they did note a significant reduction in the subgroup that was taking triptans. Moreover, a follow-up analysis by Aurora and colleagues involving 32 weeks of open-label treatment with onabotulinumtoxin A following the 24-week randomized study revealed significant reductions in acute headache days at 56 weeks.
Using anti-CGRPs without acute medication withdrawal
More recently, strong evidence is emerging about the value of using anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies without acute medication withdrawal. The findings involve 4 anti-CGRP medications.
Erenumab: A subgroup analysis of a randomized, double-blind, placebo-controlled parallel-group trial by Tepper and colleagues showed that erenumab reduced frequency of migraine at 3 months in participants with chronic migraine and medication overuse. Patients receiving either 70 or 140 mg of erenumab saw their migraine frequency reduced by an average of 6.6 days, versus 3.5 days in the placebo group.
Additionally, a significantly greater number of patients in the treatment groups stopped overusing medication, and did so early, which led to improved patient-reported outcomes. Acute migraine-specific medication treatment days were reduced by an average of 5.4 days in the 70 mg group, 4.9 days in the 140 mg contingent, and 2.9 days in those who received placebo.
Overall, consistent improvement in measures of impact, disability and health-related quality of life were seen in individuals’ treatment with erenumab.
Galcanezumab: A post-hoc analysis of pooled data from the phase 3 EVOLVE-1 and EVOLVE-2 studies, as well as the phase 3 REGAIN trial found that in participants with medication overuse, 120 mg and 240 mg doses of galcanezumab cut the number of average migraine days and decreased medication overuse. Average migraine days were lowered in EVOLVE participants by 6.26 days in the 120 mg group, 5.77 days in the 240 mg contingent, and 2.71 in those who received placebo. In REGAIN, these numbers were 4.78, 4.51, and 2.25, respectively. Average monthly medication use rates in EVOLVE were 6.2%, 7.9%, and 15.9%, respectively; in REGAIN they were 24.3%, 23.1%, and 40.6%, respectively.
Notably, though the study demonstrated galcanezumab’s efficacy in those with and without medication overuse, improvement was more pronounced in patients with medication overuse.
Fremanezumab: In an analysis by Silberstein and colleagues, significantly more patients who received quarterly or monthly injections of fremanezumab reported no medication overuse during the 3-month study, versus placebo. Specifically, 61% of participants who received monthly injections of fremanezumab and 55% of those who took quarterly injections reported no medication overuse. Among those receiving placebo, only 46% reverted to no overuse. The effect was seen as early as week 4. Additionally, among patients with medication overuse at baseline, the number of days with acute medication use was significantly lower in the treatment groups versus placebo—1.8 days lower in the quarterly group and 2.8 days in the monthly contingent.
A subsequent post-hoc analysis presented at the 2019 American Headache Society (AHS) Annual Scientific Meeting showed that the benefits were sustained over time and the medication was effective in difficult cases. Continued treatment with either quarterly or monthly dosing resulted in a reduced number of headache days, acute medication overuse headache, and headache-related disability, compared with baseline measures. Notably, about 6 in every 10 individuals with medication overuse at baseline who received fremanezumab reverted to no acute medication overuse at 6 months. This effect was maintained through 1 year of treatment.
Eptinezumab: In PROMISE-2, a post-hoc analysis of the phase 3 trials evaluating quarterly IV infusions of eptinezumab 100 mg and 300 mg, Lipton and colleagues reported that participants with chronic migraine and medication overuse experienced greater reductions in monthly migraine days during weeks 1 through 12, versus placebo (100 mg, 7.7 days; 300 mg, 8.2 days; placebo, 5.6 days). Benefits, seen as early as the day after dosing, were generally maintained or improved over 24 weeks.
Acute care medication use was reduced by about 50% in the treatment group versus roughly 25% in the placebo contingent. Most encouraging was the finding that about one-third of individuals in the treatment cohort experienced 6 months without medication overuse and below the chronic migraine diagnostic threshold; only 10% of patients who received placebo resolved in this way. Consistent improvement across patient-reported outcomes was also observed in the treatment group versus placebo.
While the studies involving topiramate, onabotulinumtoxin A, and the anti-CGRP monoclonal antibodies suggest that preventive treatment alone may effectively treat acute medical overuse and medication overuse headache, it is the data behind the anti-CGRP treatments that seem to be most compelling and causing conventional thinking to be challenged. These medications appear to be able to convert individuals with chronic migraine and medication overuse, out of overuse and back to episodic migraine. Moreover, results show they may be able to reduce acute medication use in episodic migraine, which reduces the risk of the headache sufferer transforming to chronic migraine. It is worth considering this approach in patients’ overuse acute care medication, as well as those in whom discontinuation may otherwise prove difficult without concurrent preventive treatment.
The emerging role of gepants
Availability of the so-called “gepants”—small molecule CGRP receptor agonists—is shedding additional light on management of medication overuse headache and pointing to the future. Gepants—which include ubrogepant, rimegepant, and atogepant—have been shown in early data to have a preventive effect when used regularly. Thus, it is much less likely that their use will lead to excess use and medication overuse headache.
Preclinical data demonstrated that continued use of ubrogepant does not appear to produce early or latent trigeminal sensory sensitization. Meanwhile, rimegepant, when used every other day, and as needed for acute treatment of migraine in individuals suffering from moderate-to-high frequency episodic migraine, resulted in reductions in monthly migraine days. The preventive effects appear to be rapid and sustained. And in a phase 3 trial, atogepant demonstrated efficacy at doses of 10 mg, 30 mg, and 60 mg twice a day, compared with placebo over 12 weeks.
It is important to note that the link between the gepants and medication overuse and medication overuse headache have not yet been studied. Still, it is encouraging to see that migraine frequency improves and medication use days are reduced when gepants are taken preventively. Thus, gepants could emerge as a preferred approach for acute or preventive treatment in individuals who have or are at risk of developing medication overuse headache.
The goal of treating medication overuse headache is obvious: ceasing overuse of the medication in question in an effort to return to a headache pattern that is episodic and better managed. Although guidelines suggest withdrawal of the overused medication and initiating preventive treatment, there is debate about this approach versus withdrawal alone or preventive treatment without ceasing the overused medication. A recently published randomized trial from Carlsen and colleagues evaluated 3 treatment methods: 1) withdrawal plus preventive treatment; 2) preventive treatment only; and 3) withdrawal followed by optional preventive treatment 2 months after withdrawal. Investigators found all 3 approaches effective, but participants who underwent withdrawal plus preventive care saw their headache days reduced by 12.3 days, versus 9.9 days in the preventive-only group and 8.5 days in the withdrawal/optional preventive follow-up treatment contingent. No statistically significant differences were seen between the groups in terms of migraine days, days with short-term medication use, and headache pain intensity.
Particularly noteworthy was the finding that individuals treated with withdrawal plus preventive treatment were significantly more likely to achieve remission. Specifically, nearly 75% returned to experiencing episodic headache, compared with 60% in the preventive group and 42% in the withdrawal contingent. Nearly all (97%) of those on the withdrawal plus preventive regimen were cured of medication overuse headache, versus 90% (withdrawal) and 74% (preventive).
The bottom line: Individuals undergoing withdrawal plus preventive treatment were 30% more likely to be cured of medication overuse headache. Thus, it appears that detoxification is key.
Or is it?
On the one hand…
In studies, withdrawal from the offending medication is linked with substantial improvement in headache days. Additionally, individuals who previously responded poorly to preventive treatment fared better with such treatment after detoxification.
When treating medication overuse headache using the detoxification and preventive care approach, Sun-Edelstein and colleagues outline these important steps:
- Educate your patients and their family/caregivers about the detoxification process
- Wean patient off the offending medication with a goal of complete detoxification
- Initiate preventive medical therapy or behavioral/non-drug strategies
- Establish clear limits on acute medication intake
- Arrange for regular follow-up to minimize or prevent relapse
While on the other hand…
Even though guidelines recommend detoxification, there is data supporting the concept of initiating preventive treatment without detoxification. A randomized, double-blind, placebo-controlled trial by Mei and colleagues found that 100 mg per day of topiramate led to a significant reduction in headache days and average amount of acute medication intake, versus placebo. However, treatment completion rates were low, leading Sun-Edelstein and colleagues to surmise that topiramate without detoxification would probably not have had a high success rate in practice.
Meanwhile, onabotulinumtoxin A was found in the PREEMPT trials conducted by Dodick and colleagues to reduce the number of headache days, migraine days, and moderate/severe headache days, compared with placebo, at week 24. Disappointingly, researchers found that acute medication frequency was not reduced in the overall treatment group, but they did note a significant reduction in the subgroup that was taking triptans. Moreover, a follow-up analysis by Aurora and colleagues involving 32 weeks of open-label treatment with onabotulinumtoxin A following the 24-week randomized study revealed significant reductions in acute headache days at 56 weeks.
Using anti-CGRPs without acute medication withdrawal
More recently, strong evidence is emerging about the value of using anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies without acute medication withdrawal. The findings involve 4 anti-CGRP medications.
Erenumab: A subgroup analysis of a randomized, double-blind, placebo-controlled parallel-group trial by Tepper and colleagues showed that erenumab reduced frequency of migraine at 3 months in participants with chronic migraine and medication overuse. Patients receiving either 70 or 140 mg of erenumab saw their migraine frequency reduced by an average of 6.6 days, versus 3.5 days in the placebo group.
Additionally, a significantly greater number of patients in the treatment groups stopped overusing medication, and did so early, which led to improved patient-reported outcomes. Acute migraine-specific medication treatment days were reduced by an average of 5.4 days in the 70 mg group, 4.9 days in the 140 mg contingent, and 2.9 days in those who received placebo.
Overall, consistent improvement in measures of impact, disability and health-related quality of life were seen in individuals’ treatment with erenumab.
Galcanezumab: A post-hoc analysis of pooled data from the phase 3 EVOLVE-1 and EVOLVE-2 studies, as well as the phase 3 REGAIN trial found that in participants with medication overuse, 120 mg and 240 mg doses of galcanezumab cut the number of average migraine days and decreased medication overuse. Average migraine days were lowered in EVOLVE participants by 6.26 days in the 120 mg group, 5.77 days in the 240 mg contingent, and 2.71 in those who received placebo. In REGAIN, these numbers were 4.78, 4.51, and 2.25, respectively. Average monthly medication use rates in EVOLVE were 6.2%, 7.9%, and 15.9%, respectively; in REGAIN they were 24.3%, 23.1%, and 40.6%, respectively.
Notably, though the study demonstrated galcanezumab’s efficacy in those with and without medication overuse, improvement was more pronounced in patients with medication overuse.
Fremanezumab: In an analysis by Silberstein and colleagues, significantly more patients who received quarterly or monthly injections of fremanezumab reported no medication overuse during the 3-month study, versus placebo. Specifically, 61% of participants who received monthly injections of fremanezumab and 55% of those who took quarterly injections reported no medication overuse. Among those receiving placebo, only 46% reverted to no overuse. The effect was seen as early as week 4. Additionally, among patients with medication overuse at baseline, the number of days with acute medication use was significantly lower in the treatment groups versus placebo—1.8 days lower in the quarterly group and 2.8 days in the monthly contingent.
A subsequent post-hoc analysis presented at the 2019 American Headache Society (AHS) Annual Scientific Meeting showed that the benefits were sustained over time and the medication was effective in difficult cases. Continued treatment with either quarterly or monthly dosing resulted in a reduced number of headache days, acute medication overuse headache, and headache-related disability, compared with baseline measures. Notably, about 6 in every 10 individuals with medication overuse at baseline who received fremanezumab reverted to no acute medication overuse at 6 months. This effect was maintained through 1 year of treatment.
Eptinezumab: In PROMISE-2, a post-hoc analysis of the phase 3 trials evaluating quarterly IV infusions of eptinezumab 100 mg and 300 mg, Lipton and colleagues reported that participants with chronic migraine and medication overuse experienced greater reductions in monthly migraine days during weeks 1 through 12, versus placebo (100 mg, 7.7 days; 300 mg, 8.2 days; placebo, 5.6 days). Benefits, seen as early as the day after dosing, were generally maintained or improved over 24 weeks.
Acute care medication use was reduced by about 50% in the treatment group versus roughly 25% in the placebo contingent. Most encouraging was the finding that about one-third of individuals in the treatment cohort experienced 6 months without medication overuse and below the chronic migraine diagnostic threshold; only 10% of patients who received placebo resolved in this way. Consistent improvement across patient-reported outcomes was also observed in the treatment group versus placebo.
While the studies involving topiramate, onabotulinumtoxin A, and the anti-CGRP monoclonal antibodies suggest that preventive treatment alone may effectively treat acute medical overuse and medication overuse headache, it is the data behind the anti-CGRP treatments that seem to be most compelling and causing conventional thinking to be challenged. These medications appear to be able to convert individuals with chronic migraine and medication overuse, out of overuse and back to episodic migraine. Moreover, results show they may be able to reduce acute medication use in episodic migraine, which reduces the risk of the headache sufferer transforming to chronic migraine. It is worth considering this approach in patients’ overuse acute care medication, as well as those in whom discontinuation may otherwise prove difficult without concurrent preventive treatment.
The emerging role of gepants
Availability of the so-called “gepants”—small molecule CGRP receptor agonists—is shedding additional light on management of medication overuse headache and pointing to the future. Gepants—which include ubrogepant, rimegepant, and atogepant—have been shown in early data to have a preventive effect when used regularly. Thus, it is much less likely that their use will lead to excess use and medication overuse headache.
Preclinical data demonstrated that continued use of ubrogepant does not appear to produce early or latent trigeminal sensory sensitization. Meanwhile, rimegepant, when used every other day, and as needed for acute treatment of migraine in individuals suffering from moderate-to-high frequency episodic migraine, resulted in reductions in monthly migraine days. The preventive effects appear to be rapid and sustained. And in a phase 3 trial, atogepant demonstrated efficacy at doses of 10 mg, 30 mg, and 60 mg twice a day, compared with placebo over 12 weeks.
It is important to note that the link between the gepants and medication overuse and medication overuse headache have not yet been studied. Still, it is encouraging to see that migraine frequency improves and medication use days are reduced when gepants are taken preventively. Thus, gepants could emerge as a preferred approach for acute or preventive treatment in individuals who have or are at risk of developing medication overuse headache.
The goal of treating medication overuse headache is obvious: ceasing overuse of the medication in question in an effort to return to a headache pattern that is episodic and better managed. Although guidelines suggest withdrawal of the overused medication and initiating preventive treatment, there is debate about this approach versus withdrawal alone or preventive treatment without ceasing the overused medication. A recently published randomized trial from Carlsen and colleagues evaluated 3 treatment methods: 1) withdrawal plus preventive treatment; 2) preventive treatment only; and 3) withdrawal followed by optional preventive treatment 2 months after withdrawal. Investigators found all 3 approaches effective, but participants who underwent withdrawal plus preventive care saw their headache days reduced by 12.3 days, versus 9.9 days in the preventive-only group and 8.5 days in the withdrawal/optional preventive follow-up treatment contingent. No statistically significant differences were seen between the groups in terms of migraine days, days with short-term medication use, and headache pain intensity.
Particularly noteworthy was the finding that individuals treated with withdrawal plus preventive treatment were significantly more likely to achieve remission. Specifically, nearly 75% returned to experiencing episodic headache, compared with 60% in the preventive group and 42% in the withdrawal contingent. Nearly all (97%) of those on the withdrawal plus preventive regimen were cured of medication overuse headache, versus 90% (withdrawal) and 74% (preventive).
The bottom line: Individuals undergoing withdrawal plus preventive treatment were 30% more likely to be cured of medication overuse headache. Thus, it appears that detoxification is key.
Or is it?
On the one hand…
In studies, withdrawal from the offending medication is linked with substantial improvement in headache days. Additionally, individuals who previously responded poorly to preventive treatment fared better with such treatment after detoxification.
When treating medication overuse headache using the detoxification and preventive care approach, Sun-Edelstein and colleagues outline these important steps:
- Educate your patients and their family/caregivers about the detoxification process
- Wean patient off the offending medication with a goal of complete detoxification
- Initiate preventive medical therapy or behavioral/non-drug strategies
- Establish clear limits on acute medication intake
- Arrange for regular follow-up to minimize or prevent relapse
While on the other hand…
Even though guidelines recommend detoxification, there is data supporting the concept of initiating preventive treatment without detoxification. A randomized, double-blind, placebo-controlled trial by Mei and colleagues found that 100 mg per day of topiramate led to a significant reduction in headache days and average amount of acute medication intake, versus placebo. However, treatment completion rates were low, leading Sun-Edelstein and colleagues to surmise that topiramate without detoxification would probably not have had a high success rate in practice.
Meanwhile, onabotulinumtoxin A was found in the PREEMPT trials conducted by Dodick and colleagues to reduce the number of headache days, migraine days, and moderate/severe headache days, compared with placebo, at week 24. Disappointingly, researchers found that acute medication frequency was not reduced in the overall treatment group, but they did note a significant reduction in the subgroup that was taking triptans. Moreover, a follow-up analysis by Aurora and colleagues involving 32 weeks of open-label treatment with onabotulinumtoxin A following the 24-week randomized study revealed significant reductions in acute headache days at 56 weeks.
Using anti-CGRPs without acute medication withdrawal
More recently, strong evidence is emerging about the value of using anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies without acute medication withdrawal. The findings involve 4 anti-CGRP medications.
Erenumab: A subgroup analysis of a randomized, double-blind, placebo-controlled parallel-group trial by Tepper and colleagues showed that erenumab reduced frequency of migraine at 3 months in participants with chronic migraine and medication overuse. Patients receiving either 70 or 140 mg of erenumab saw their migraine frequency reduced by an average of 6.6 days, versus 3.5 days in the placebo group.
Additionally, a significantly greater number of patients in the treatment groups stopped overusing medication, and did so early, which led to improved patient-reported outcomes. Acute migraine-specific medication treatment days were reduced by an average of 5.4 days in the 70 mg group, 4.9 days in the 140 mg contingent, and 2.9 days in those who received placebo.
Overall, consistent improvement in measures of impact, disability and health-related quality of life were seen in individuals’ treatment with erenumab.
Galcanezumab: A post-hoc analysis of pooled data from the phase 3 EVOLVE-1 and EVOLVE-2 studies, as well as the phase 3 REGAIN trial found that in participants with medication overuse, 120 mg and 240 mg doses of galcanezumab cut the number of average migraine days and decreased medication overuse. Average migraine days were lowered in EVOLVE participants by 6.26 days in the 120 mg group, 5.77 days in the 240 mg contingent, and 2.71 in those who received placebo. In REGAIN, these numbers were 4.78, 4.51, and 2.25, respectively. Average monthly medication use rates in EVOLVE were 6.2%, 7.9%, and 15.9%, respectively; in REGAIN they were 24.3%, 23.1%, and 40.6%, respectively.
Notably, though the study demonstrated galcanezumab’s efficacy in those with and without medication overuse, improvement was more pronounced in patients with medication overuse.
Fremanezumab: In an analysis by Silberstein and colleagues, significantly more patients who received quarterly or monthly injections of fremanezumab reported no medication overuse during the 3-month study, versus placebo. Specifically, 61% of participants who received monthly injections of fremanezumab and 55% of those who took quarterly injections reported no medication overuse. Among those receiving placebo, only 46% reverted to no overuse. The effect was seen as early as week 4. Additionally, among patients with medication overuse at baseline, the number of days with acute medication use was significantly lower in the treatment groups versus placebo—1.8 days lower in the quarterly group and 2.8 days in the monthly contingent.
A subsequent post-hoc analysis presented at the 2019 American Headache Society (AHS) Annual Scientific Meeting showed that the benefits were sustained over time and the medication was effective in difficult cases. Continued treatment with either quarterly or monthly dosing resulted in a reduced number of headache days, acute medication overuse headache, and headache-related disability, compared with baseline measures. Notably, about 6 in every 10 individuals with medication overuse at baseline who received fremanezumab reverted to no acute medication overuse at 6 months. This effect was maintained through 1 year of treatment.
Eptinezumab: In PROMISE-2, a post-hoc analysis of the phase 3 trials evaluating quarterly IV infusions of eptinezumab 100 mg and 300 mg, Lipton and colleagues reported that participants with chronic migraine and medication overuse experienced greater reductions in monthly migraine days during weeks 1 through 12, versus placebo (100 mg, 7.7 days; 300 mg, 8.2 days; placebo, 5.6 days). Benefits, seen as early as the day after dosing, were generally maintained or improved over 24 weeks.
Acute care medication use was reduced by about 50% in the treatment group versus roughly 25% in the placebo contingent. Most encouraging was the finding that about one-third of individuals in the treatment cohort experienced 6 months without medication overuse and below the chronic migraine diagnostic threshold; only 10% of patients who received placebo resolved in this way. Consistent improvement across patient-reported outcomes was also observed in the treatment group versus placebo.
While the studies involving topiramate, onabotulinumtoxin A, and the anti-CGRP monoclonal antibodies suggest that preventive treatment alone may effectively treat acute medical overuse and medication overuse headache, it is the data behind the anti-CGRP treatments that seem to be most compelling and causing conventional thinking to be challenged. These medications appear to be able to convert individuals with chronic migraine and medication overuse, out of overuse and back to episodic migraine. Moreover, results show they may be able to reduce acute medication use in episodic migraine, which reduces the risk of the headache sufferer transforming to chronic migraine. It is worth considering this approach in patients’ overuse acute care medication, as well as those in whom discontinuation may otherwise prove difficult without concurrent preventive treatment.
The emerging role of gepants
Availability of the so-called “gepants”—small molecule CGRP receptor agonists—is shedding additional light on management of medication overuse headache and pointing to the future. Gepants—which include ubrogepant, rimegepant, and atogepant—have been shown in early data to have a preventive effect when used regularly. Thus, it is much less likely that their use will lead to excess use and medication overuse headache.
Preclinical data demonstrated that continued use of ubrogepant does not appear to produce early or latent trigeminal sensory sensitization. Meanwhile, rimegepant, when used every other day, and as needed for acute treatment of migraine in individuals suffering from moderate-to-high frequency episodic migraine, resulted in reductions in monthly migraine days. The preventive effects appear to be rapid and sustained. And in a phase 3 trial, atogepant demonstrated efficacy at doses of 10 mg, 30 mg, and 60 mg twice a day, compared with placebo over 12 weeks.
It is important to note that the link between the gepants and medication overuse and medication overuse headache have not yet been studied. Still, it is encouraging to see that migraine frequency improves and medication use days are reduced when gepants are taken preventively. Thus, gepants could emerge as a preferred approach for acute or preventive treatment in individuals who have or are at risk of developing medication overuse headache.
Risk factors identified for late seizure relapse after epilepsy surgery
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
FROM EPILEPSIA
High-dose methotrexate of no CNS benefit for patients with high-risk DLBCL
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM BLOOD ADVANCES
Patients with CLL have significantly reduced response to COVID-19 vaccine
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
FROM BLOOD ADVANCES
Race, ethnicity, and socioeconomics are often barriers to migraine care
, according to a study published in the April issue of Headache. People of African descent and Latinx ethnicity tend to fare worse than other people of color and their White counterparts.
“It should be shocking to neurologists and other clinicians who care for migraine patients how few are able to successfully traverse the barriers to achieve an accurate diagnosis and proper, evidence-based, acute and preventative treatment,” commented Peter McAllister, MD, medical director at the New England Institute for Neurology and Headache and chief medical officer for clinical research at Ki Clinical Research in Stamford, Conn. Dr. McAllister was not involved in this study.
Assessing barriers to care
Researchers designed the study with the primary objective of estimating the number of patients with migraines with unmet clinical needs and who were impacted by four preidentified barriers to care. To evaluate their objective, researchers conducted a longitudinal, Internet-based survey known as the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. They collected data over 1 year examining a cohort of patients that mimicked the diverse demographics of the U.S. population. Researchers conducted longitudinal assessments every 3 months for 15 months, incorporating cross-sectional analyses that surveyed health care use, family burden, and comorbidities or endophenotypes.
Eligible enrollees were 18 years of age or older.
Researchers identified four barriers that hindered patient outcomes, and they served as the primary outcomes of the studies. They were:
- Health care provider consultations. Investigators used study participants’ responses to the following question during their interactions with their health care providers to help evaluate the quality of their consultation experience: “What type of doctor is currently managing your headaches?” Researchers included data from patients whose practitioners fit the description of those they deemed best suited to address ongoing headache challenges. These medical professionals included general practitioners, family physicians, internal medicine doctors, nurse practitioners, physician assistants, neurologists, pain specialists, headache specialists, and obstetrician-gynecologists.
- Diagnosis. Carefully evaluating patients’ responses to a series of questions helped researchers gauge the accuracy of diagnosis. Questions included: “Have you ever been diagnosed by a doctor or other health professional with any of the following types of headaches?” Respondents were also given a list of options that provided additional context around their headaches and were encouraged to select all appropriate responses. The list included a fictional response option of “citrene headache” to determine incorrect responses. For this study, researchers deemed it necessary to recognize a chronic migraine diagnosis to ensure that patients received appropriate treatment.
- Minimally appropriate pharmacologic treatment. Researchers used the following question to determine whether patients’ chronic migraine and episodic migraine were being managed with the least amount of pharmacological treatment necessary. “Which of these medications (if any) are you currently using (or typically keep on hand) to treat your headaches when you have them?” Researchers defined “minimally appropriate acute pharmacologic treatment” as the use of any prescription nonsteroidal anti-inflammatory drug (NSAID), triptan, ergotamine derivative, or isometheptene.
- Avoidance of medication overuse. The study authors pointed out the sometimes nebulous process of characterizing the appropriate use of preventative medication in patients with episodic migraines as “not straightforward” for some patients because not all patients require preventive treatment. Study participants were required to report having received any form of preventative therapy, defined as pharmacological therapies approved by guidelines and supported by data. Such therapies included various antiseizure medication, antidepressants (for example, doxepin, venlafaxine, duloxetine, amitriptyline, imipramine, nortriptyline, and desvenlafaxine), antihypertensives, and toxin injections. Treatments such as behavioral and neuromodulatory therapies were excluded from the list.
According to lead author Dawn C. Buse, PhD, of the department of neurology at Albert Einstein College of Medicine, New York, acute medication overuse provides an important modifiable target for intervention and recommends that clinicians use the opportunity to optimize migraine care by reducing the patients’ reliance on acute therapies. Taking such initiatives to decrease medication overuse is especially important in communities of color, who are more likely to overuse medications for migraines.
Patients with higher income levels were more likely to overcome each barrier. People of African, African American, or multiracial descent were more prone to overuse of medications to manage their migraines.
Of the 489,537 respondents invited to participate in the CaMEO study, 16,879 qualified for inclusion. Slightly more than half of the respondents (n = 9,184 [54.7%]) had a migraine-related disability (MIDAS) score of 6 or greater – an indicator of disability that is least mild in nature. Most patients who had episodic migraines or chronic migraines (86.2%) had some form of health insurance coverage (n = 9.184; 84.1%; P = .048). Of those patients who were insured, 7,930 patients experienced episodic migraine (86.3%) and the remainder had chronic migraine (n = 1,254; 13.7%). Higher-income patients were more likely to traverse barriers to care. While patients of African descent had higher consultation rates, they also had higher rates of acute medication overuse.
Patients with chronic migraine were more likely to be older than patients with episodic migraine (41.0 vs. 39.6 years; P = .0001) and female (83.0% vs. 79.0%; P = .001), and White (84.5% vs. 79.1%; P < .001). Similarly, patients with chronic migraine were more likely to have a higher mean body mass index (29.8 kg/m2 vs. 28.9 kg/m2; P < .001) and lower rates of full- or part-time employment (56.8% vs. 67.1%; P < .001), and were less likely to have a 4-year degree (64.8 vs. 55.6; P < .001) and annual household incomes below $75,000 (72.6% vs. 64.6%; P < .001). Approximately three-quarters of the patients with episodic migraine (75.7%; 1655/2187) and one-third of patients with chronic migraine (32.8%; 168/512) received accurate diagnoses.
The data uncovered an association with acute medication overuse. Among current consulters who had received an accurate diagnosis and minimally adequate treatment, medication overuse rates were highest among those reporting two or more races (53%) and Blacks and African Americans (45%) and lowest among Whites (33%) and those categorized as “other” race (32%). Ethnic and cultural differences in headache literacy may contribute to differences in medication overuse.
Strategies to improve outcomes
Both Dr. Buse and Dr. McAllister see the value advocacy and education offer in helping to improve outcomes in marginalized communities and other groups negatively impacted by various barriers.
“Patient advocacy and outreach are key here, especially in those traditionally underrepresented in the migraine space, such as men, people of color, blue-collar workers, etc.,” Dr. McAllister noted.
Dr. Buse emphasized the importance of education for patients and health care professionals alike. “A large percentage of people who meet criteria for migraine in the U.S. do not seek care or possibly even know that they have migraines,” Dr. Buse said. “This finding underscores the importance of public health education about migraine as well as well as providing migraine support, education, and resources to health care professionals on the front lines.”
Other strategies recommended by Dr, Buse to ease the impact of barriers include encouraging patient discussion, setting up time for follow-up appointments and education, referring patients for neurological and other specialty consults when warranted, reviewing essential lifestyle habits for migraine management, and creating personalized, mutually agreed-upon treatment plans.
Dr. Buse has received support and honoraria from AbbVie, Amgen, Avanir, Biohaven, Eli Lilly, and Promius.
, according to a study published in the April issue of Headache. People of African descent and Latinx ethnicity tend to fare worse than other people of color and their White counterparts.
“It should be shocking to neurologists and other clinicians who care for migraine patients how few are able to successfully traverse the barriers to achieve an accurate diagnosis and proper, evidence-based, acute and preventative treatment,” commented Peter McAllister, MD, medical director at the New England Institute for Neurology and Headache and chief medical officer for clinical research at Ki Clinical Research in Stamford, Conn. Dr. McAllister was not involved in this study.
Assessing barriers to care
Researchers designed the study with the primary objective of estimating the number of patients with migraines with unmet clinical needs and who were impacted by four preidentified barriers to care. To evaluate their objective, researchers conducted a longitudinal, Internet-based survey known as the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. They collected data over 1 year examining a cohort of patients that mimicked the diverse demographics of the U.S. population. Researchers conducted longitudinal assessments every 3 months for 15 months, incorporating cross-sectional analyses that surveyed health care use, family burden, and comorbidities or endophenotypes.
Eligible enrollees were 18 years of age or older.
Researchers identified four barriers that hindered patient outcomes, and they served as the primary outcomes of the studies. They were:
- Health care provider consultations. Investigators used study participants’ responses to the following question during their interactions with their health care providers to help evaluate the quality of their consultation experience: “What type of doctor is currently managing your headaches?” Researchers included data from patients whose practitioners fit the description of those they deemed best suited to address ongoing headache challenges. These medical professionals included general practitioners, family physicians, internal medicine doctors, nurse practitioners, physician assistants, neurologists, pain specialists, headache specialists, and obstetrician-gynecologists.
- Diagnosis. Carefully evaluating patients’ responses to a series of questions helped researchers gauge the accuracy of diagnosis. Questions included: “Have you ever been diagnosed by a doctor or other health professional with any of the following types of headaches?” Respondents were also given a list of options that provided additional context around their headaches and were encouraged to select all appropriate responses. The list included a fictional response option of “citrene headache” to determine incorrect responses. For this study, researchers deemed it necessary to recognize a chronic migraine diagnosis to ensure that patients received appropriate treatment.
- Minimally appropriate pharmacologic treatment. Researchers used the following question to determine whether patients’ chronic migraine and episodic migraine were being managed with the least amount of pharmacological treatment necessary. “Which of these medications (if any) are you currently using (or typically keep on hand) to treat your headaches when you have them?” Researchers defined “minimally appropriate acute pharmacologic treatment” as the use of any prescription nonsteroidal anti-inflammatory drug (NSAID), triptan, ergotamine derivative, or isometheptene.
- Avoidance of medication overuse. The study authors pointed out the sometimes nebulous process of characterizing the appropriate use of preventative medication in patients with episodic migraines as “not straightforward” for some patients because not all patients require preventive treatment. Study participants were required to report having received any form of preventative therapy, defined as pharmacological therapies approved by guidelines and supported by data. Such therapies included various antiseizure medication, antidepressants (for example, doxepin, venlafaxine, duloxetine, amitriptyline, imipramine, nortriptyline, and desvenlafaxine), antihypertensives, and toxin injections. Treatments such as behavioral and neuromodulatory therapies were excluded from the list.
According to lead author Dawn C. Buse, PhD, of the department of neurology at Albert Einstein College of Medicine, New York, acute medication overuse provides an important modifiable target for intervention and recommends that clinicians use the opportunity to optimize migraine care by reducing the patients’ reliance on acute therapies. Taking such initiatives to decrease medication overuse is especially important in communities of color, who are more likely to overuse medications for migraines.
Patients with higher income levels were more likely to overcome each barrier. People of African, African American, or multiracial descent were more prone to overuse of medications to manage their migraines.
Of the 489,537 respondents invited to participate in the CaMEO study, 16,879 qualified for inclusion. Slightly more than half of the respondents (n = 9,184 [54.7%]) had a migraine-related disability (MIDAS) score of 6 or greater – an indicator of disability that is least mild in nature. Most patients who had episodic migraines or chronic migraines (86.2%) had some form of health insurance coverage (n = 9.184; 84.1%; P = .048). Of those patients who were insured, 7,930 patients experienced episodic migraine (86.3%) and the remainder had chronic migraine (n = 1,254; 13.7%). Higher-income patients were more likely to traverse barriers to care. While patients of African descent had higher consultation rates, they also had higher rates of acute medication overuse.
Patients with chronic migraine were more likely to be older than patients with episodic migraine (41.0 vs. 39.6 years; P = .0001) and female (83.0% vs. 79.0%; P = .001), and White (84.5% vs. 79.1%; P < .001). Similarly, patients with chronic migraine were more likely to have a higher mean body mass index (29.8 kg/m2 vs. 28.9 kg/m2; P < .001) and lower rates of full- or part-time employment (56.8% vs. 67.1%; P < .001), and were less likely to have a 4-year degree (64.8 vs. 55.6; P < .001) and annual household incomes below $75,000 (72.6% vs. 64.6%; P < .001). Approximately three-quarters of the patients with episodic migraine (75.7%; 1655/2187) and one-third of patients with chronic migraine (32.8%; 168/512) received accurate diagnoses.
The data uncovered an association with acute medication overuse. Among current consulters who had received an accurate diagnosis and minimally adequate treatment, medication overuse rates were highest among those reporting two or more races (53%) and Blacks and African Americans (45%) and lowest among Whites (33%) and those categorized as “other” race (32%). Ethnic and cultural differences in headache literacy may contribute to differences in medication overuse.
Strategies to improve outcomes
Both Dr. Buse and Dr. McAllister see the value advocacy and education offer in helping to improve outcomes in marginalized communities and other groups negatively impacted by various barriers.
“Patient advocacy and outreach are key here, especially in those traditionally underrepresented in the migraine space, such as men, people of color, blue-collar workers, etc.,” Dr. McAllister noted.
Dr. Buse emphasized the importance of education for patients and health care professionals alike. “A large percentage of people who meet criteria for migraine in the U.S. do not seek care or possibly even know that they have migraines,” Dr. Buse said. “This finding underscores the importance of public health education about migraine as well as well as providing migraine support, education, and resources to health care professionals on the front lines.”
Other strategies recommended by Dr, Buse to ease the impact of barriers include encouraging patient discussion, setting up time for follow-up appointments and education, referring patients for neurological and other specialty consults when warranted, reviewing essential lifestyle habits for migraine management, and creating personalized, mutually agreed-upon treatment plans.
Dr. Buse has received support and honoraria from AbbVie, Amgen, Avanir, Biohaven, Eli Lilly, and Promius.
, according to a study published in the April issue of Headache. People of African descent and Latinx ethnicity tend to fare worse than other people of color and their White counterparts.
“It should be shocking to neurologists and other clinicians who care for migraine patients how few are able to successfully traverse the barriers to achieve an accurate diagnosis and proper, evidence-based, acute and preventative treatment,” commented Peter McAllister, MD, medical director at the New England Institute for Neurology and Headache and chief medical officer for clinical research at Ki Clinical Research in Stamford, Conn. Dr. McAllister was not involved in this study.
Assessing barriers to care
Researchers designed the study with the primary objective of estimating the number of patients with migraines with unmet clinical needs and who were impacted by four preidentified barriers to care. To evaluate their objective, researchers conducted a longitudinal, Internet-based survey known as the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. They collected data over 1 year examining a cohort of patients that mimicked the diverse demographics of the U.S. population. Researchers conducted longitudinal assessments every 3 months for 15 months, incorporating cross-sectional analyses that surveyed health care use, family burden, and comorbidities or endophenotypes.
Eligible enrollees were 18 years of age or older.
Researchers identified four barriers that hindered patient outcomes, and they served as the primary outcomes of the studies. They were:
- Health care provider consultations. Investigators used study participants’ responses to the following question during their interactions with their health care providers to help evaluate the quality of their consultation experience: “What type of doctor is currently managing your headaches?” Researchers included data from patients whose practitioners fit the description of those they deemed best suited to address ongoing headache challenges. These medical professionals included general practitioners, family physicians, internal medicine doctors, nurse practitioners, physician assistants, neurologists, pain specialists, headache specialists, and obstetrician-gynecologists.
- Diagnosis. Carefully evaluating patients’ responses to a series of questions helped researchers gauge the accuracy of diagnosis. Questions included: “Have you ever been diagnosed by a doctor or other health professional with any of the following types of headaches?” Respondents were also given a list of options that provided additional context around their headaches and were encouraged to select all appropriate responses. The list included a fictional response option of “citrene headache” to determine incorrect responses. For this study, researchers deemed it necessary to recognize a chronic migraine diagnosis to ensure that patients received appropriate treatment.
- Minimally appropriate pharmacologic treatment. Researchers used the following question to determine whether patients’ chronic migraine and episodic migraine were being managed with the least amount of pharmacological treatment necessary. “Which of these medications (if any) are you currently using (or typically keep on hand) to treat your headaches when you have them?” Researchers defined “minimally appropriate acute pharmacologic treatment” as the use of any prescription nonsteroidal anti-inflammatory drug (NSAID), triptan, ergotamine derivative, or isometheptene.
- Avoidance of medication overuse. The study authors pointed out the sometimes nebulous process of characterizing the appropriate use of preventative medication in patients with episodic migraines as “not straightforward” for some patients because not all patients require preventive treatment. Study participants were required to report having received any form of preventative therapy, defined as pharmacological therapies approved by guidelines and supported by data. Such therapies included various antiseizure medication, antidepressants (for example, doxepin, venlafaxine, duloxetine, amitriptyline, imipramine, nortriptyline, and desvenlafaxine), antihypertensives, and toxin injections. Treatments such as behavioral and neuromodulatory therapies were excluded from the list.
According to lead author Dawn C. Buse, PhD, of the department of neurology at Albert Einstein College of Medicine, New York, acute medication overuse provides an important modifiable target for intervention and recommends that clinicians use the opportunity to optimize migraine care by reducing the patients’ reliance on acute therapies. Taking such initiatives to decrease medication overuse is especially important in communities of color, who are more likely to overuse medications for migraines.
Patients with higher income levels were more likely to overcome each barrier. People of African, African American, or multiracial descent were more prone to overuse of medications to manage their migraines.
Of the 489,537 respondents invited to participate in the CaMEO study, 16,879 qualified for inclusion. Slightly more than half of the respondents (n = 9,184 [54.7%]) had a migraine-related disability (MIDAS) score of 6 or greater – an indicator of disability that is least mild in nature. Most patients who had episodic migraines or chronic migraines (86.2%) had some form of health insurance coverage (n = 9.184; 84.1%; P = .048). Of those patients who were insured, 7,930 patients experienced episodic migraine (86.3%) and the remainder had chronic migraine (n = 1,254; 13.7%). Higher-income patients were more likely to traverse barriers to care. While patients of African descent had higher consultation rates, they also had higher rates of acute medication overuse.
Patients with chronic migraine were more likely to be older than patients with episodic migraine (41.0 vs. 39.6 years; P = .0001) and female (83.0% vs. 79.0%; P = .001), and White (84.5% vs. 79.1%; P < .001). Similarly, patients with chronic migraine were more likely to have a higher mean body mass index (29.8 kg/m2 vs. 28.9 kg/m2; P < .001) and lower rates of full- or part-time employment (56.8% vs. 67.1%; P < .001), and were less likely to have a 4-year degree (64.8 vs. 55.6; P < .001) and annual household incomes below $75,000 (72.6% vs. 64.6%; P < .001). Approximately three-quarters of the patients with episodic migraine (75.7%; 1655/2187) and one-third of patients with chronic migraine (32.8%; 168/512) received accurate diagnoses.
The data uncovered an association with acute medication overuse. Among current consulters who had received an accurate diagnosis and minimally adequate treatment, medication overuse rates were highest among those reporting two or more races (53%) and Blacks and African Americans (45%) and lowest among Whites (33%) and those categorized as “other” race (32%). Ethnic and cultural differences in headache literacy may contribute to differences in medication overuse.
Strategies to improve outcomes
Both Dr. Buse and Dr. McAllister see the value advocacy and education offer in helping to improve outcomes in marginalized communities and other groups negatively impacted by various barriers.
“Patient advocacy and outreach are key here, especially in those traditionally underrepresented in the migraine space, such as men, people of color, blue-collar workers, etc.,” Dr. McAllister noted.
Dr. Buse emphasized the importance of education for patients and health care professionals alike. “A large percentage of people who meet criteria for migraine in the U.S. do not seek care or possibly even know that they have migraines,” Dr. Buse said. “This finding underscores the importance of public health education about migraine as well as well as providing migraine support, education, and resources to health care professionals on the front lines.”
Other strategies recommended by Dr, Buse to ease the impact of barriers include encouraging patient discussion, setting up time for follow-up appointments and education, referring patients for neurological and other specialty consults when warranted, reviewing essential lifestyle habits for migraine management, and creating personalized, mutually agreed-upon treatment plans.
Dr. Buse has received support and honoraria from AbbVie, Amgen, Avanir, Biohaven, Eli Lilly, and Promius.
FROM HEADACHE
Headache on the Hill goes virtual
Participants in the Alliance for Headache Disorders Advocacy session requested federal funding for headache research and treatment. While patients told their stories, a noted advocate said headache is “an eminently solvable problem, but the urgency is now.”
It is going to take more than a pandemic to stop key headache advocacy stakeholders from raising awareness of the devastating impact of migraine and cluster headache and to motivate Congress to act.
With COVID-19 still very much a part of our lives, the Alliance for Headache Disorders Advocacy (AHDA)—a nonprofit dedicated to advocating for equitable policies for people with headache disorders—moved forward with its annual Headache on the Hill advocacy day, which took place virtually for the first time via videoconferencing on March 23, 2021.
While participants missed the opportunity to travel to Washington to meet with key legislators face-to-face, optimists saw it as a chance to involve more patients, providers, researchers, and caregivers who otherwise would not be able to participate. Indeed, more were involved than ever before: 217 individuals from 47 states and 178 Congressional districts attended, meeting with influential lawmakers, including Senator Patrick Leahy (D-VT), chair of the Appropriations Committee; Senator Richard Shelby (R-AL), vice chair of the Appropriations Committee; Rep. Rose DeLauro (D-CT), chair of the House Committee on Appropriations; Senator Jon Tester (D-MT), chair of Senate Committee on Veterans’ Affairs; Senator Jerry Moran (R-KS), ranking member of the Senate Committee on Veterans’ Affairs; Senator Patty Murray (D-WA), chair of the Senate HELP Committee; and Senator Richard Burr (R-NC), ranking member of the Senate HELP Committee.
I have had the privilege of being a part of Headache on the Hill for 13 years and was pleased to participate in this year’s virtual event. Though the setting was different, our mission remained the same: to make our important legislative requests (“asks”) of as many offices in Congress as possible. This year, we had 2 asks that aim to improve headache research and access to treatment, especially for our Veterans:
- Increased research funding: The group requested that the National Institutes of Health (NIH) Helping to End Addiction Long-Term (HEAL) initiative focus on headache disorders to reduce disease burden and opioid prescribing. This would make more funding available for headache research.
- Improved treatment access: The group also asked Congress to fully fund Veterans Health Administration (VHA) Headache Disorders Centers of Excellence (HCoE), facilitating equitable access to care for disabled veterans. This would double the number of VA Centers of Excellence to treat headache disorders in our veterans.
Of course, getting results means more than simply asking. The request to our congresspeople and their staff is more likely to succeed if it is well-reasoned and backed by evidence; and the Headache on the Hill contingent delivered on these requirements.
Why Congress should direct HEAL to focus on headache disorders:
- Headache disorders are extraordinarily burdensome. As most of us know (but not all legislators are aware), 60 million Americans suffer from migraine headache; it is the second leading cause of disability lifetime in the world.1 Additionally, cluster headache is thought to be the most severe type of pain humans can experience.2
- There is a critical need for more effective and safer treatments for headache disorders. Opioid use is known to worsen migraine frequency and severity for some and make medications for headache less effective.3 Guidelines uniformly recommend against treating migraine with opioids; yet somehow 10% of migraine sufferers actively use opioids,4 and nearly 60% receive opioids during visits to the emergency room.5
- NIH has underfunded research on headache disorders. NIH has not prioritized programs for headache disorders research despite the fact that since 2009, 17 appropriations report language statements have strongly urged NIH to do so.6 In fact, headache is the least-funded research area among the most burdensome diseases.7,8 Instead, other important disorders were funded, even though Headache on the Hill advocacy arranged for the report language for headache.
- Statutory authority for the HEAL initiative calls for disease burden to be a “crucial consideration” in prioritizing research programs. Less than 1% of HEAL grants have been for headache disorders research.10 If disease burden was used as the only gauge for funding, NIH investment for migraine research would likely be 15 times higher than the roughly $20 million that has historically been allocated.9 We hope our work this year will get us where we need to be.
Why Congress should fully fund VHA Headache Disorders Centers of Excellence
- Headache disorders are a major health issue for veterans. Some 350,000 Global War on Terror (GWOT) veterans have sustained traumatic brain injuries. Many of them experience headaches. In fact, research shows that half of these veterans reported 15 or more headache days per month 4 to 11 years after sustaining traumatic brain injury. Nine of every 10 veterans met the criteria for migraine.10 Moreover, 3 million GWOT veterans have been exposed to toxic open burn pits.11 These individuals have been found to be twice as likely to experience functional limitations due to migraine than those who did not have burn pit duties.12
- Headache Centers of Excellence (HCoEs) work. In 2018, $10 million was appropriated to establish at least 5 HCoEs that provided 1) comprehensive direct patient specialized headache medicine care within the VHA; 2) consultation and referral specialized headache care centers within the VHA; 3) education and training of VHA healthcare providers in headache medicine; and 4) research to improve the quality of headache disorders care for veterans and civilians.13
- Fourteen sites now exist, and success continues to be demonstrated. Last year more than 400,000 veterans sought specialty care for headache disorders from the VHA.14 However, only half of these vets are within reasonable reach of a HCoE.
The asks
Armed with this evidence, we made specific asks of the House and Senate with respect to annual appropriations spending bills:
- Legislators were asked to sign on to a letter or send their own letter to officials on the House and Senate Labor, Health and Human Services, Education, and Related Agencies appropriations subcommittees to allocate $50 million from the HEAL initiative for headache disorders research in fiscal year 2022.
- Similarly, lawmakers were asked to sign onto a letter or send their own letter to members of the Military Construction and Veterans Affairs subcommittee to appropriate $25 million to fund a doubling of HCoEs from 14 to 28 to improve access to those seeking care for headache disorders.
Stories from Americans nationwide
Headache on the Hill is about more than just presenting evidence and making requests. If that were the case, there is a pretty good chance that, before long, legislators would be looking at their watches, checking their smartphones, and flashing knowing glances at their aides in an effort to cut things short. However, humanizing the topic by sharing stories of the toll migraine and cluster headaches take on individuals is compelling testimony that hopefully will lead to meaningful action and positive outcomes. Here is a sampling of the stories told during and after the Headache on the Hill session:
- Rachel Koh and Ronetta Stokes: Koh registered for both the 2019 and 2020 Headache on the Hill sessions, only to be forced to cancel due to migraine attacks. But this year, according to the American Migraine Foundation, she was able to participate virtually and tell her representatives why increased funding was important for her, as well as veterans, including her father and uncle. Meanwhile, Stokes, a first-time participant, said she was struck by the conversations she had with legislators. Most knew someone with migraine, and some were sufferers themselves. “The more we share and spread the word, the sooner we can end the stigma,” Stokes told the American Migraine Foundation.
- Mia Maysack: Maysack wrote about her experience in a column for Pain News Network. “I live with both migraine disease and cluster headaches, which are called ‘suicide headaches’ for good reason,” she wrote. “There’s no limit to the chaos, interruption, inconvenience, and discomfort these conditions have caused in my life, requiring my full-time attention just to manage the symptoms. The difficult experiences I and countless others have faced in seeking, finding, and attempting different forms of treatment is why I continue to advocate—even when I don't feel up to it.” Maysack added that although it is relatively easy for her to receive a medication prescription for her condition, she’d like to see more consideration given to treatments such as water therapy, massage, oxygen, and mindful meditation.
- Chloe Vruno: Vruno, a 21-year-old college student, has suffered with migraine since the age of 15. “Some days are worse than others,” she noted in an article in her local newspaper, the Steuben County, IN Herald Republican. “Most days I have to push through a migraine to make it to class, but some days are so severe that I cannot make it to classes. On days I cannot make it, I use my accommodation for attendance flexibility, or now with COVID, I Zoom into class from my room.” Vruno wanted to make her representatives aware of these types of disruptions, a regular occurrence for migraine sufferers like her. This was her second Headache on the Hill event, and she found lawmakers whom she spoke with to be “extremely attentive, engaged, and excited.”
“A moral imperative”
Stories like these from regular individuals across the United States who suffer from headache disorders go a long way in convincing legislators to act. It also helps to tap into a celebrity’s endorsement when you can. Headache on the Hill did not disappoint in this regard, with Jon Stewart, the former host of The Daily Show, appearing as a special guest during a policy panel discussion on chronic headache disorders and toxic exposure. The session, which took place virtually as part of Headache on the Hill, featured Stewart, a national advocate for service personnel with toxic exposures, and Rep. Mark Takano (D-CA), who delivered the keynote address. The panel discussion included first responders, veterans, and clinicians.
Stewart summed up the sentiments of all Headache on the Hill stakeholders this way: “This is an eminently solvable problem, but the urgency is now. People will continue to suffer needlessly if we don’t get this done. It is a moral imperative that we pass a bill on presumption as soon as possible.”
I always enjoy going to Washington on cold days in February to be part of Headache on the Hill, and I hope we will be back in-person next year. We have tripled the number of attendees over the last 13 years and have a higher percentage of great patients and advocates now. I have to give a special thanks to Dr. Bob Shapiro, Professor of Neurology at the University of Vermont, who started and has guided this phenomenal effort over the years, to Dr. Chris Gottschalk, Professor of Neurology at Yale, who is gradually taking over the reins, and to Katie MacDonald who runs the entire show, even though she suffers from chronic migraine on a daily basis.
1. Global Health Data Exchange. Global Burden of Disease Study 2019 (GBD 2019) Data Resources. http://ghdx.healthdata.org/gbd-2019. Accessed April 12, 2021.
2.Burish MJ, Pearson SM, RE Shapiro, et al. Cluster headache is one of the most intensely painful human conditions: Results from the International Cluster Headache Questionnaire. Headache. 2021;61:117-124.
3. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
4. Lipton RB, Buse DB, Dodick DW, et al. Burden of increasing opioid use in the treatment of migraine: Results from the Migraine in America Symptoms and Treatment Study. Headache. 2020;61:103-116.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: An analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Shapiro RE. What will it take to move the needle for headache disorders? An advocacy perspective. Headache. 2020;60:2059-2077.
7. NIH RePORT. Report on NIH funding vs. global burden of disease. https://report.nih.gov/report-nih-funding-vs-global-burden-disease. Accessed April 12, 2021.
8. NIH RePORT. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/funding/categorical-spending#/. Published February 24, 2020. Accessed April 12, 2021.
9. National Institutes of Health. Funded projects. https://heal.nih.gov/funding/awarded. Updated March 18, 2020. Accessed April 12, 2021.
10. Couch JR, Stewart KE. Headache prevalence at 4-11 years after deployment-related traumatic brain injury in veterans of Iraq and Afghanistan wars and comparison to controls: A matched case-controlled study. Headache 2016;56:1004-1021.
11. Dr. Richard A. Stone, Acting Under Secretary for Health. Message to Staff-Airborne Hazards and Open Burn Pit Registry. https://players.brightcove.net/2851863979001/default_default/index.html?videoId=6228317154001. Published February 2021. Accessed April 12, 2021.
12. US Department of Veterans Affairs. Report on Data from the Airborne Hazards and Open Burn Pit (AH&OBP) Registry. https://www.publichealth.va.gov/docs/exposures/va-ahobp-registry-data-report-june2015.pdf#. Published June 2015. Accessed April 12, 2021.
13. US Government Publishing Office. Military construction, Veterans Affairs, and related agencies appropriation bill, 2018. https://www.appropriations.senate.gov/imo/media/doc/FY2018%20MiliCon-VA%20Bill%20S1557.pdf. Published July 13, 2017. Accessed April 12, 2021.
14. Fenton BT, Lindsey H, Grinberg AS, et al. Presentation given at: 62nd Annual Scientific Meeting American Headache Society- Prevalence of Headache and Comorbidities Among Men and Women Veterans Across the Veterans Health Administration – a 10‐year Cohort Study. VA Connecticut Healthcare System, West Haven, CT; Yale School of Medicine, West Haven, CT; 3Yeshiva University, Bronx, NY. https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854. Published June 13, 2020. Accessed April 12, 2021.
Participants in the Alliance for Headache Disorders Advocacy session requested federal funding for headache research and treatment. While patients told their stories, a noted advocate said headache is “an eminently solvable problem, but the urgency is now.”
It is going to take more than a pandemic to stop key headache advocacy stakeholders from raising awareness of the devastating impact of migraine and cluster headache and to motivate Congress to act.
With COVID-19 still very much a part of our lives, the Alliance for Headache Disorders Advocacy (AHDA)—a nonprofit dedicated to advocating for equitable policies for people with headache disorders—moved forward with its annual Headache on the Hill advocacy day, which took place virtually for the first time via videoconferencing on March 23, 2021.
While participants missed the opportunity to travel to Washington to meet with key legislators face-to-face, optimists saw it as a chance to involve more patients, providers, researchers, and caregivers who otherwise would not be able to participate. Indeed, more were involved than ever before: 217 individuals from 47 states and 178 Congressional districts attended, meeting with influential lawmakers, including Senator Patrick Leahy (D-VT), chair of the Appropriations Committee; Senator Richard Shelby (R-AL), vice chair of the Appropriations Committee; Rep. Rose DeLauro (D-CT), chair of the House Committee on Appropriations; Senator Jon Tester (D-MT), chair of Senate Committee on Veterans’ Affairs; Senator Jerry Moran (R-KS), ranking member of the Senate Committee on Veterans’ Affairs; Senator Patty Murray (D-WA), chair of the Senate HELP Committee; and Senator Richard Burr (R-NC), ranking member of the Senate HELP Committee.
I have had the privilege of being a part of Headache on the Hill for 13 years and was pleased to participate in this year’s virtual event. Though the setting was different, our mission remained the same: to make our important legislative requests (“asks”) of as many offices in Congress as possible. This year, we had 2 asks that aim to improve headache research and access to treatment, especially for our Veterans:
- Increased research funding: The group requested that the National Institutes of Health (NIH) Helping to End Addiction Long-Term (HEAL) initiative focus on headache disorders to reduce disease burden and opioid prescribing. This would make more funding available for headache research.
- Improved treatment access: The group also asked Congress to fully fund Veterans Health Administration (VHA) Headache Disorders Centers of Excellence (HCoE), facilitating equitable access to care for disabled veterans. This would double the number of VA Centers of Excellence to treat headache disorders in our veterans.
Of course, getting results means more than simply asking. The request to our congresspeople and their staff is more likely to succeed if it is well-reasoned and backed by evidence; and the Headache on the Hill contingent delivered on these requirements.
Why Congress should direct HEAL to focus on headache disorders:
- Headache disorders are extraordinarily burdensome. As most of us know (but not all legislators are aware), 60 million Americans suffer from migraine headache; it is the second leading cause of disability lifetime in the world.1 Additionally, cluster headache is thought to be the most severe type of pain humans can experience.2
- There is a critical need for more effective and safer treatments for headache disorders. Opioid use is known to worsen migraine frequency and severity for some and make medications for headache less effective.3 Guidelines uniformly recommend against treating migraine with opioids; yet somehow 10% of migraine sufferers actively use opioids,4 and nearly 60% receive opioids during visits to the emergency room.5
- NIH has underfunded research on headache disorders. NIH has not prioritized programs for headache disorders research despite the fact that since 2009, 17 appropriations report language statements have strongly urged NIH to do so.6 In fact, headache is the least-funded research area among the most burdensome diseases.7,8 Instead, other important disorders were funded, even though Headache on the Hill advocacy arranged for the report language for headache.
- Statutory authority for the HEAL initiative calls for disease burden to be a “crucial consideration” in prioritizing research programs. Less than 1% of HEAL grants have been for headache disorders research.10 If disease burden was used as the only gauge for funding, NIH investment for migraine research would likely be 15 times higher than the roughly $20 million that has historically been allocated.9 We hope our work this year will get us where we need to be.
Why Congress should fully fund VHA Headache Disorders Centers of Excellence
- Headache disorders are a major health issue for veterans. Some 350,000 Global War on Terror (GWOT) veterans have sustained traumatic brain injuries. Many of them experience headaches. In fact, research shows that half of these veterans reported 15 or more headache days per month 4 to 11 years after sustaining traumatic brain injury. Nine of every 10 veterans met the criteria for migraine.10 Moreover, 3 million GWOT veterans have been exposed to toxic open burn pits.11 These individuals have been found to be twice as likely to experience functional limitations due to migraine than those who did not have burn pit duties.12
- Headache Centers of Excellence (HCoEs) work. In 2018, $10 million was appropriated to establish at least 5 HCoEs that provided 1) comprehensive direct patient specialized headache medicine care within the VHA; 2) consultation and referral specialized headache care centers within the VHA; 3) education and training of VHA healthcare providers in headache medicine; and 4) research to improve the quality of headache disorders care for veterans and civilians.13
- Fourteen sites now exist, and success continues to be demonstrated. Last year more than 400,000 veterans sought specialty care for headache disorders from the VHA.14 However, only half of these vets are within reasonable reach of a HCoE.
The asks
Armed with this evidence, we made specific asks of the House and Senate with respect to annual appropriations spending bills:
- Legislators were asked to sign on to a letter or send their own letter to officials on the House and Senate Labor, Health and Human Services, Education, and Related Agencies appropriations subcommittees to allocate $50 million from the HEAL initiative for headache disorders research in fiscal year 2022.
- Similarly, lawmakers were asked to sign onto a letter or send their own letter to members of the Military Construction and Veterans Affairs subcommittee to appropriate $25 million to fund a doubling of HCoEs from 14 to 28 to improve access to those seeking care for headache disorders.
Stories from Americans nationwide
Headache on the Hill is about more than just presenting evidence and making requests. If that were the case, there is a pretty good chance that, before long, legislators would be looking at their watches, checking their smartphones, and flashing knowing glances at their aides in an effort to cut things short. However, humanizing the topic by sharing stories of the toll migraine and cluster headaches take on individuals is compelling testimony that hopefully will lead to meaningful action and positive outcomes. Here is a sampling of the stories told during and after the Headache on the Hill session:
- Rachel Koh and Ronetta Stokes: Koh registered for both the 2019 and 2020 Headache on the Hill sessions, only to be forced to cancel due to migraine attacks. But this year, according to the American Migraine Foundation, she was able to participate virtually and tell her representatives why increased funding was important for her, as well as veterans, including her father and uncle. Meanwhile, Stokes, a first-time participant, said she was struck by the conversations she had with legislators. Most knew someone with migraine, and some were sufferers themselves. “The more we share and spread the word, the sooner we can end the stigma,” Stokes told the American Migraine Foundation.
- Mia Maysack: Maysack wrote about her experience in a column for Pain News Network. “I live with both migraine disease and cluster headaches, which are called ‘suicide headaches’ for good reason,” she wrote. “There’s no limit to the chaos, interruption, inconvenience, and discomfort these conditions have caused in my life, requiring my full-time attention just to manage the symptoms. The difficult experiences I and countless others have faced in seeking, finding, and attempting different forms of treatment is why I continue to advocate—even when I don't feel up to it.” Maysack added that although it is relatively easy for her to receive a medication prescription for her condition, she’d like to see more consideration given to treatments such as water therapy, massage, oxygen, and mindful meditation.
- Chloe Vruno: Vruno, a 21-year-old college student, has suffered with migraine since the age of 15. “Some days are worse than others,” she noted in an article in her local newspaper, the Steuben County, IN Herald Republican. “Most days I have to push through a migraine to make it to class, but some days are so severe that I cannot make it to classes. On days I cannot make it, I use my accommodation for attendance flexibility, or now with COVID, I Zoom into class from my room.” Vruno wanted to make her representatives aware of these types of disruptions, a regular occurrence for migraine sufferers like her. This was her second Headache on the Hill event, and she found lawmakers whom she spoke with to be “extremely attentive, engaged, and excited.”
“A moral imperative”
Stories like these from regular individuals across the United States who suffer from headache disorders go a long way in convincing legislators to act. It also helps to tap into a celebrity’s endorsement when you can. Headache on the Hill did not disappoint in this regard, with Jon Stewart, the former host of The Daily Show, appearing as a special guest during a policy panel discussion on chronic headache disorders and toxic exposure. The session, which took place virtually as part of Headache on the Hill, featured Stewart, a national advocate for service personnel with toxic exposures, and Rep. Mark Takano (D-CA), who delivered the keynote address. The panel discussion included first responders, veterans, and clinicians.
Stewart summed up the sentiments of all Headache on the Hill stakeholders this way: “This is an eminently solvable problem, but the urgency is now. People will continue to suffer needlessly if we don’t get this done. It is a moral imperative that we pass a bill on presumption as soon as possible.”
I always enjoy going to Washington on cold days in February to be part of Headache on the Hill, and I hope we will be back in-person next year. We have tripled the number of attendees over the last 13 years and have a higher percentage of great patients and advocates now. I have to give a special thanks to Dr. Bob Shapiro, Professor of Neurology at the University of Vermont, who started and has guided this phenomenal effort over the years, to Dr. Chris Gottschalk, Professor of Neurology at Yale, who is gradually taking over the reins, and to Katie MacDonald who runs the entire show, even though she suffers from chronic migraine on a daily basis.
Participants in the Alliance for Headache Disorders Advocacy session requested federal funding for headache research and treatment. While patients told their stories, a noted advocate said headache is “an eminently solvable problem, but the urgency is now.”
It is going to take more than a pandemic to stop key headache advocacy stakeholders from raising awareness of the devastating impact of migraine and cluster headache and to motivate Congress to act.
With COVID-19 still very much a part of our lives, the Alliance for Headache Disorders Advocacy (AHDA)—a nonprofit dedicated to advocating for equitable policies for people with headache disorders—moved forward with its annual Headache on the Hill advocacy day, which took place virtually for the first time via videoconferencing on March 23, 2021.
While participants missed the opportunity to travel to Washington to meet with key legislators face-to-face, optimists saw it as a chance to involve more patients, providers, researchers, and caregivers who otherwise would not be able to participate. Indeed, more were involved than ever before: 217 individuals from 47 states and 178 Congressional districts attended, meeting with influential lawmakers, including Senator Patrick Leahy (D-VT), chair of the Appropriations Committee; Senator Richard Shelby (R-AL), vice chair of the Appropriations Committee; Rep. Rose DeLauro (D-CT), chair of the House Committee on Appropriations; Senator Jon Tester (D-MT), chair of Senate Committee on Veterans’ Affairs; Senator Jerry Moran (R-KS), ranking member of the Senate Committee on Veterans’ Affairs; Senator Patty Murray (D-WA), chair of the Senate HELP Committee; and Senator Richard Burr (R-NC), ranking member of the Senate HELP Committee.
I have had the privilege of being a part of Headache on the Hill for 13 years and was pleased to participate in this year’s virtual event. Though the setting was different, our mission remained the same: to make our important legislative requests (“asks”) of as many offices in Congress as possible. This year, we had 2 asks that aim to improve headache research and access to treatment, especially for our Veterans:
- Increased research funding: The group requested that the National Institutes of Health (NIH) Helping to End Addiction Long-Term (HEAL) initiative focus on headache disorders to reduce disease burden and opioid prescribing. This would make more funding available for headache research.
- Improved treatment access: The group also asked Congress to fully fund Veterans Health Administration (VHA) Headache Disorders Centers of Excellence (HCoE), facilitating equitable access to care for disabled veterans. This would double the number of VA Centers of Excellence to treat headache disorders in our veterans.
Of course, getting results means more than simply asking. The request to our congresspeople and their staff is more likely to succeed if it is well-reasoned and backed by evidence; and the Headache on the Hill contingent delivered on these requirements.
Why Congress should direct HEAL to focus on headache disorders:
- Headache disorders are extraordinarily burdensome. As most of us know (but not all legislators are aware), 60 million Americans suffer from migraine headache; it is the second leading cause of disability lifetime in the world.1 Additionally, cluster headache is thought to be the most severe type of pain humans can experience.2
- There is a critical need for more effective and safer treatments for headache disorders. Opioid use is known to worsen migraine frequency and severity for some and make medications for headache less effective.3 Guidelines uniformly recommend against treating migraine with opioids; yet somehow 10% of migraine sufferers actively use opioids,4 and nearly 60% receive opioids during visits to the emergency room.5
- NIH has underfunded research on headache disorders. NIH has not prioritized programs for headache disorders research despite the fact that since 2009, 17 appropriations report language statements have strongly urged NIH to do so.6 In fact, headache is the least-funded research area among the most burdensome diseases.7,8 Instead, other important disorders were funded, even though Headache on the Hill advocacy arranged for the report language for headache.
- Statutory authority for the HEAL initiative calls for disease burden to be a “crucial consideration” in prioritizing research programs. Less than 1% of HEAL grants have been for headache disorders research.10 If disease burden was used as the only gauge for funding, NIH investment for migraine research would likely be 15 times higher than the roughly $20 million that has historically been allocated.9 We hope our work this year will get us where we need to be.
Why Congress should fully fund VHA Headache Disorders Centers of Excellence
- Headache disorders are a major health issue for veterans. Some 350,000 Global War on Terror (GWOT) veterans have sustained traumatic brain injuries. Many of them experience headaches. In fact, research shows that half of these veterans reported 15 or more headache days per month 4 to 11 years after sustaining traumatic brain injury. Nine of every 10 veterans met the criteria for migraine.10 Moreover, 3 million GWOT veterans have been exposed to toxic open burn pits.11 These individuals have been found to be twice as likely to experience functional limitations due to migraine than those who did not have burn pit duties.12
- Headache Centers of Excellence (HCoEs) work. In 2018, $10 million was appropriated to establish at least 5 HCoEs that provided 1) comprehensive direct patient specialized headache medicine care within the VHA; 2) consultation and referral specialized headache care centers within the VHA; 3) education and training of VHA healthcare providers in headache medicine; and 4) research to improve the quality of headache disorders care for veterans and civilians.13
- Fourteen sites now exist, and success continues to be demonstrated. Last year more than 400,000 veterans sought specialty care for headache disorders from the VHA.14 However, only half of these vets are within reasonable reach of a HCoE.
The asks
Armed with this evidence, we made specific asks of the House and Senate with respect to annual appropriations spending bills:
- Legislators were asked to sign on to a letter or send their own letter to officials on the House and Senate Labor, Health and Human Services, Education, and Related Agencies appropriations subcommittees to allocate $50 million from the HEAL initiative for headache disorders research in fiscal year 2022.
- Similarly, lawmakers were asked to sign onto a letter or send their own letter to members of the Military Construction and Veterans Affairs subcommittee to appropriate $25 million to fund a doubling of HCoEs from 14 to 28 to improve access to those seeking care for headache disorders.
Stories from Americans nationwide
Headache on the Hill is about more than just presenting evidence and making requests. If that were the case, there is a pretty good chance that, before long, legislators would be looking at their watches, checking their smartphones, and flashing knowing glances at their aides in an effort to cut things short. However, humanizing the topic by sharing stories of the toll migraine and cluster headaches take on individuals is compelling testimony that hopefully will lead to meaningful action and positive outcomes. Here is a sampling of the stories told during and after the Headache on the Hill session:
- Rachel Koh and Ronetta Stokes: Koh registered for both the 2019 and 2020 Headache on the Hill sessions, only to be forced to cancel due to migraine attacks. But this year, according to the American Migraine Foundation, she was able to participate virtually and tell her representatives why increased funding was important for her, as well as veterans, including her father and uncle. Meanwhile, Stokes, a first-time participant, said she was struck by the conversations she had with legislators. Most knew someone with migraine, and some were sufferers themselves. “The more we share and spread the word, the sooner we can end the stigma,” Stokes told the American Migraine Foundation.
- Mia Maysack: Maysack wrote about her experience in a column for Pain News Network. “I live with both migraine disease and cluster headaches, which are called ‘suicide headaches’ for good reason,” she wrote. “There’s no limit to the chaos, interruption, inconvenience, and discomfort these conditions have caused in my life, requiring my full-time attention just to manage the symptoms. The difficult experiences I and countless others have faced in seeking, finding, and attempting different forms of treatment is why I continue to advocate—even when I don't feel up to it.” Maysack added that although it is relatively easy for her to receive a medication prescription for her condition, she’d like to see more consideration given to treatments such as water therapy, massage, oxygen, and mindful meditation.
- Chloe Vruno: Vruno, a 21-year-old college student, has suffered with migraine since the age of 15. “Some days are worse than others,” she noted in an article in her local newspaper, the Steuben County, IN Herald Republican. “Most days I have to push through a migraine to make it to class, but some days are so severe that I cannot make it to classes. On days I cannot make it, I use my accommodation for attendance flexibility, or now with COVID, I Zoom into class from my room.” Vruno wanted to make her representatives aware of these types of disruptions, a regular occurrence for migraine sufferers like her. This was her second Headache on the Hill event, and she found lawmakers whom she spoke with to be “extremely attentive, engaged, and excited.”
“A moral imperative”
Stories like these from regular individuals across the United States who suffer from headache disorders go a long way in convincing legislators to act. It also helps to tap into a celebrity’s endorsement when you can. Headache on the Hill did not disappoint in this regard, with Jon Stewart, the former host of The Daily Show, appearing as a special guest during a policy panel discussion on chronic headache disorders and toxic exposure. The session, which took place virtually as part of Headache on the Hill, featured Stewart, a national advocate for service personnel with toxic exposures, and Rep. Mark Takano (D-CA), who delivered the keynote address. The panel discussion included first responders, veterans, and clinicians.
Stewart summed up the sentiments of all Headache on the Hill stakeholders this way: “This is an eminently solvable problem, but the urgency is now. People will continue to suffer needlessly if we don’t get this done. It is a moral imperative that we pass a bill on presumption as soon as possible.”
I always enjoy going to Washington on cold days in February to be part of Headache on the Hill, and I hope we will be back in-person next year. We have tripled the number of attendees over the last 13 years and have a higher percentage of great patients and advocates now. I have to give a special thanks to Dr. Bob Shapiro, Professor of Neurology at the University of Vermont, who started and has guided this phenomenal effort over the years, to Dr. Chris Gottschalk, Professor of Neurology at Yale, who is gradually taking over the reins, and to Katie MacDonald who runs the entire show, even though she suffers from chronic migraine on a daily basis.
1. Global Health Data Exchange. Global Burden of Disease Study 2019 (GBD 2019) Data Resources. http://ghdx.healthdata.org/gbd-2019. Accessed April 12, 2021.
2.Burish MJ, Pearson SM, RE Shapiro, et al. Cluster headache is one of the most intensely painful human conditions: Results from the International Cluster Headache Questionnaire. Headache. 2021;61:117-124.
3. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
4. Lipton RB, Buse DB, Dodick DW, et al. Burden of increasing opioid use in the treatment of migraine: Results from the Migraine in America Symptoms and Treatment Study. Headache. 2020;61:103-116.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: An analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Shapiro RE. What will it take to move the needle for headache disorders? An advocacy perspective. Headache. 2020;60:2059-2077.
7. NIH RePORT. Report on NIH funding vs. global burden of disease. https://report.nih.gov/report-nih-funding-vs-global-burden-disease. Accessed April 12, 2021.
8. NIH RePORT. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/funding/categorical-spending#/. Published February 24, 2020. Accessed April 12, 2021.
9. National Institutes of Health. Funded projects. https://heal.nih.gov/funding/awarded. Updated March 18, 2020. Accessed April 12, 2021.
10. Couch JR, Stewart KE. Headache prevalence at 4-11 years after deployment-related traumatic brain injury in veterans of Iraq and Afghanistan wars and comparison to controls: A matched case-controlled study. Headache 2016;56:1004-1021.
11. Dr. Richard A. Stone, Acting Under Secretary for Health. Message to Staff-Airborne Hazards and Open Burn Pit Registry. https://players.brightcove.net/2851863979001/default_default/index.html?videoId=6228317154001. Published February 2021. Accessed April 12, 2021.
12. US Department of Veterans Affairs. Report on Data from the Airborne Hazards and Open Burn Pit (AH&OBP) Registry. https://www.publichealth.va.gov/docs/exposures/va-ahobp-registry-data-report-june2015.pdf#. Published June 2015. Accessed April 12, 2021.
13. US Government Publishing Office. Military construction, Veterans Affairs, and related agencies appropriation bill, 2018. https://www.appropriations.senate.gov/imo/media/doc/FY2018%20MiliCon-VA%20Bill%20S1557.pdf. Published July 13, 2017. Accessed April 12, 2021.
14. Fenton BT, Lindsey H, Grinberg AS, et al. Presentation given at: 62nd Annual Scientific Meeting American Headache Society- Prevalence of Headache and Comorbidities Among Men and Women Veterans Across the Veterans Health Administration – a 10‐year Cohort Study. VA Connecticut Healthcare System, West Haven, CT; Yale School of Medicine, West Haven, CT; 3Yeshiva University, Bronx, NY. https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854. Published June 13, 2020. Accessed April 12, 2021.
1. Global Health Data Exchange. Global Burden of Disease Study 2019 (GBD 2019) Data Resources. http://ghdx.healthdata.org/gbd-2019. Accessed April 12, 2021.
2.Burish MJ, Pearson SM, RE Shapiro, et al. Cluster headache is one of the most intensely painful human conditions: Results from the International Cluster Headache Questionnaire. Headache. 2021;61:117-124.
3. Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821-1828.
4. Lipton RB, Buse DB, Dodick DW, et al. Burden of increasing opioid use in the treatment of migraine: Results from the Migraine in America Symptoms and Treatment Study. Headache. 2020;61:103-116.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: An analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Shapiro RE. What will it take to move the needle for headache disorders? An advocacy perspective. Headache. 2020;60:2059-2077.
7. NIH RePORT. Report on NIH funding vs. global burden of disease. https://report.nih.gov/report-nih-funding-vs-global-burden-disease. Accessed April 12, 2021.
8. NIH RePORT. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/funding/categorical-spending#/. Published February 24, 2020. Accessed April 12, 2021.
9. National Institutes of Health. Funded projects. https://heal.nih.gov/funding/awarded. Updated March 18, 2020. Accessed April 12, 2021.
10. Couch JR, Stewart KE. Headache prevalence at 4-11 years after deployment-related traumatic brain injury in veterans of Iraq and Afghanistan wars and comparison to controls: A matched case-controlled study. Headache 2016;56:1004-1021.
11. Dr. Richard A. Stone, Acting Under Secretary for Health. Message to Staff-Airborne Hazards and Open Burn Pit Registry. https://players.brightcove.net/2851863979001/default_default/index.html?videoId=6228317154001. Published February 2021. Accessed April 12, 2021.
12. US Department of Veterans Affairs. Report on Data from the Airborne Hazards and Open Burn Pit (AH&OBP) Registry. https://www.publichealth.va.gov/docs/exposures/va-ahobp-registry-data-report-june2015.pdf#. Published June 2015. Accessed April 12, 2021.
13. US Government Publishing Office. Military construction, Veterans Affairs, and related agencies appropriation bill, 2018. https://www.appropriations.senate.gov/imo/media/doc/FY2018%20MiliCon-VA%20Bill%20S1557.pdf. Published July 13, 2017. Accessed April 12, 2021.
14. Fenton BT, Lindsey H, Grinberg AS, et al. Presentation given at: 62nd Annual Scientific Meeting American Headache Society- Prevalence of Headache and Comorbidities Among Men and Women Veterans Across the Veterans Health Administration – a 10‐year Cohort Study. VA Connecticut Healthcare System, West Haven, CT; Yale School of Medicine, West Haven, CT; 3Yeshiva University, Bronx, NY. https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854. Published June 13, 2020. Accessed April 12, 2021.
Delayed Coronary Vasospasm in a Patient with Metastatic Gastric Cancer Receiving FOLFOX Therapy
A 40-year-old man with stage IV gastric adenocarcinoma was found to have coronary artery vasospasm in the setting of recent 5-fluorouracil administration.
Coronary artery vasospasm is a rare but well-known adverse effect of 5-fluorouracil (5-FU) that can be life threatening if unrecognized. Patients typically present with anginal chest pain and ST elevations on electrocardiogram (ECG) without atherosclerotic disease on coronary angiography. This phenomenon typically occurs during or shortly after infusion and resolves within hours to days after cessation of 5-FU.
In this report, we present an unusual case of coronary artery vasospasm that intermittently recurred for 25 days following 5-FU treatment in a 40-year-old male with stage IV gastric adenocarcinoma. We also review the literature on typical presentation and risk factors for 5-FU-induced coronary vasospasm, findings on coronary angiography, and management options.
5-FU is an IV administered antimetabolite chemotherapy commonly used to treat solid tumors, including gastrointestinal, pancreatic, breast, and head and neck tumors. 5-FU inhibits thymidylate synthase, which reduces levels of thymidine, a key pyrimidine nucleoside required for DNA replication within tumor cells.1 For several decades, 5-FU has remained one of the first-line drugs for colorectal cancer because it may be curative. It is the third most commonly used chemotherapy in the world and is included on the World Health Organization’s list of essential medicines.2
Cardiotoxicity occurs in 1.2 to 18% of patients who receive 5-FU therapy.3 Although there is variability in presentation for acute cardiotoxicity from 5-FU, including sudden death, angina pectoris, myocardial infarction, and ventricular arrhythmias, the mechanism most commonly implicated is coronary artery vasospasm.3 The direct observation of active coronary artery vasospasm during left heart catheterization is rare due its transient nature; however, several case studies have managed to demonstrate this.4,5 The pathophysiology of 5-FU-induced cardiotoxicity is unknown, but adverse effects on cardiac microvasculature, myocyte metabolism, platelet aggregation, and coronary vasoconstriction have all been proposed.3,6In the current case, we present a patient with stage IV gastric adenocarcinoma who complained of chest pain during hospitalization and was found to have coronary artery vasospasm in the setting of recent 5-FU administration. Following coronary angiography that showed a lack of atherosclerotic disease, the patient continued to experience episodes of chest pain with ST elevations on ECG that recurred despite cessation of 5-FU and repeated administration of vasodilatory medications.
Case Presentation
A male aged 40 years was admitted to the hospital for abdominal pain, with initial imaging concerning for partial small bowel obstruction. His history included recently diagnosed stage IV gastric adenocarcinoma complicated by peritoneal carcinomatosis status post initiation of infusional FOLFOX-4 (5-FU, leucovorin, and oxaliplatin) 11 days prior. The patient was treated for small bowel obstruction. However, several days after admission, he developed nonpleuritic, substernal chest pain unrelated to exertion and unrelieved by rest. The patient reported no known risk factors, family history, or personal history of coronary artery disease. Baseline echocardiography and ECG performed several months prior showed normal left ventricular function without ischemic findings.
Physical examination at the time of chest pain revealed a heart rate of 140 beats/min. The remainder of his vital signs were within normal range. There were no murmurs, rubs, gallops, or additional heart sounds heard on cardiac auscultation. Chest pain was not reproducible to palpation or positional in nature. An ECG demonstrated dynamic inferolateral ST elevations with reciprocal changes in leads I and aVL (Figure 1). A bedside echocardiogram showed hypokinesis of the septal wall. Troponin-I returned below the detectable level.
The patient was taken for emergent coronary catheterization, which demonstrated patent epicardial coronary arteries without atherosclerosis, a left ventricular ejection fraction of 60%, and a right dominant heart (Figures 2 and 3). Ventriculogram showed normal wall motion. Repeat troponin-I several hours after catheterization was again below detectable levels.
Given the patient’s acute onset of chest pain and inferolateral ST elevations seen on ECG, the working diagnosis prior to coronary catherization was acute coronary syndrome. The differential diagnosis included other causes of life-threatening chest pain, including pulmonary embolism, pneumonia, aortic dissection, myopericarditis, pericardial effusion, cardiac tamponade, or coronary artery vasospasm. Computed tomography (CT) angiography of the chest was not consistent with pulmonary embolism or other acute cardiopulmonary process. Based on findings from coronary angiography and recent exposure to 5-FU, as well as resolution followed by recurrence of chest pain and ECG changes over weeks, the most likely diagnosis after coronary catheterization was coronary artery vasospasm.
Treatment
Following catheterization, the patient returned to the medical intensive care unit, where he continued to report intermittent episodes of chest pain with ST elevations. In the following days, he was started on isosorbide mononitrate 150 mg daily and amlodipine 10 mg daily. Although these vasodilatory agents reduced the frequency of his chest pain episodes, intermittent chest pain associated with ST elevations on ECG continued even with maximal doses of isosorbide mononitrate and amlodipine. Administration of sublingual nitroglycerin during chest pain episodes effectively relieved his chest pain. Given the severity and frequency of the patient’s chest pain, the oncology consult team recommended foregoing further chemotherapeutic treatment with 5-FU.
Outcome
Despite holding 5-FU throughout the patient’s hospitalization and treating the patient with antianginal mediations, frequent chest pain episodes associated with ST elevations continued to recur until 25 days after his last treatment with 5-FU (Figure 4). The patient eventually expired during this hospital stay due to cancer-related complications.
Discussion
Coronary artery vasospasm is a well-known complication of 5-FU that can be life threatening if unrecognized.6-8 As seen in our case, patients typically present with anginal chest pain relieved with nitrates and ST elevations on ECG in the absence of occlusive macrovascular disease on coronary angiography.
A unique aspect of 5-FU is its variability in dose and frequency of administration across chemotherapeutic regimens. Particularly, 5-FU can be administered in daily intravenous bolus doses or as a continuous infusion for a protracted length of time. The spectrum of toxicity from 5-FU differs depending on the dose and frequency of administration. Bolus administration of 5-FU, for example, is thought to be associated with a higher rate of myelosuppression, while infusional administration of 5-FU is thought to be associated with a higher rate of cardiotoxicity and a higher tumor response rate.9
Most cases of coronary vasospasm occur either during infusion of 5-FU or within hours to days after completion. The median time of presentation for 5-FU-induced coronary artery vasospasm is about 12 hours postinfusion, while the most delayed presentation reported in the literature is 72 hours postinfusion.6,8 Delayed presentation of vasospasm may result from the release of potent vasoactive metabolites of 5-FU that accumulate over time; therefore, infusional administration may accentuate this effect.6,9 Remarkably, our patient’s chest pain episodes persisted for 25 days despite treatment with anti-anginal medications, highlighting the extent to which infusional 5-FU can produce a delay in adverse cardiotoxic effects and the importance of ongoing clinical vigilance after 5-FU exposure.
Vasospasm alone does not completely explain the spectrum of cardiac toxicity attributed to 5-FU administration. As in our case, coronary angiography during symptomatic episodes often fails to demonstrate coronary vasospasm.8 Additionally, ergonovine, an alkaloid agent used to assess coronary vasomotor function, failed to induce coronary vasospasm in some patients with suspected 5-FU-induced cardiac toxicity.10 The lack of vasospasm in some patients with 5-FU-induced cardiac toxicity suggests multiple independent effects of 5-FU on cardiac tissue that are poorly understood.
In the absence of obvious macrovascular effects, there also may be a deleterious effect of 5-FU on the coronary microvasculature that may result in coronary artery vasospasm. Though coronary microvasculature cannot be directly visualized, observation of slowed coronary blood velocity indicates a reduction in microvascular flow.8 Thus, the failure to observe epicardial coronary vasospasm in our patient does not preclude a vasospastic pathology.
The heterogeneous presentation of coronary artery vasospasm demands consideration of other disease processes such as atherosclerotic coronary artery disease, pericarditis, myopericarditis, primary arrythmias, and stress-induced cardiomyopathy, all of which have been described in association with 5-FU administration.8 A 12-lead ECG should be performed during a suspected attack. An ECG will typically demonstrate ST elevations corresponding to spasm of the involved vessel. Reciprocal ST depressions in the contralateral leads also may be seen. ECG may be useful in the acute setting to identify regional wall motion abnormalities or to rule out pericardial effusion as a cause. Cardiac biomarkers such as troponin-I, -C, and creatine kinase typically are less useful because they are often normal, even in known coronary artery vasospasm.11
Coronary angiography during an episode may show a localized region of vasospasm in an epicardial artery. Diffuse multivessel vasospasm does occur, and the location of vasospasm may change, but these events are rare. Under normal circumstances, provocative testing involving angiography with administration of acetylcholine, ergot agents, or hyperventilation can be performed. However, this type of investigation should be limited to specialized centers and should not be performed in the acute phase of the disease.12
Treatment of suspected coronary vasospasm in patients receiving 5-FU involves stopping the infusion and administering calcium channel blockers or oral nitrates to relieve anginal symptoms.13 5-FU-induced coronary artery vasospasm has a 90% rate of recurrence with subsequent infusions.8 If possible, alternate chemotherapy regimens should be considered once coronary artery vasospasm has been identified.14,15 If further 5-FU use is required, or if benefits are deemed to outweigh risks, infusions should be given in an inpatient setting with continuous cardiac monitoring.16
Calcium channel blockers and oral nitrates have been found to produce benefit in patients in acute settings; however, there is little evidence to attest to their effectiveness as prophylactic agents in those receiving 5-FU. Some reports demonstrate episodes where both calcium channel blockers and oral nitrates failed to prevent subsequent vasospasms.17 Although this was the case for our patient, short-acting sublingual nitroglycerin seemed to be effective in reducing the frequency of anginal symptoms.
Long-term outcomes have not been well investigated for patients with 5-FU-induced coronary vasospasm. However, many case reports show improvements in left ventricular function between 8 and 15 days after discontinuation of 5-FU.7,10 Although this would be a valuable topic for further research, the rarity of this phenomenon creates limitations.
Conclusions
5-FU is a first-line chemotherapy for gastrointestinal cancers that is generally well tolerated but may be associated with potentially life-threatening cardiotoxic effects, of which coronary artery vasospasm is the most common. Coronary artery vasospasm presents with anginal chest pain and ST elevations on ECG that can be indistinguishable from acute coronary syndrome. Diagnosis requires cardiac catheterization, which will reveal patent coronary arteries. Infusional administration of 5-FU may be more likely to produce late cardiotoxic effects and a longer period of persistent symptoms, necessitating close monitoring for days or even weeks from last administration of 5-FU. Coronary artery vasospasm should be treated with anti-anginal medications, though varying degrees of effectiveness can be seen; clinicians should remain vigilant for recurrent episodes of chest pain despite treatment.
1. Wacker A, Lersch C, Scherpinski U, Reindl L, Seyfarth M. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology. 2003;65(2):108-112. doi:10.1159/000072334
2. World Health Organization Model List of Essential Medicines, 21st List, 2019. Accessed April 14, 2021. https://apps.who.int/iris/rest/bitstreams/1237479/retrieve
3. Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58(4):487-493. doi:10.1007/s00280-005-0178-1
4. Shoemaker LK, Arora U, Rocha Lima CM. 5-fluorouracil-induced coronary vasospasm. Cancer Control. 2004;11(1):46-49. doi:10.1177/107327480401100207
5. Luwaert RJ, Descamps O, Majois F, Chaudron JM, Beauduin M. Coronary artery spasm induced by 5-fluorouracil. Eur Heart J. 1991;12(3):468-470. doi:10.1093/oxfordjournals.eurheartj.a059919
6. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191-202. doi:10.1517/14740330902733961
7. Patel B, Kloner RA, Ensley J, Al-Sarraf M, Kish J, Wynne J. 5-Fluorouracil cardiotoxicity: left ventricular dysfunction and effect of coronary vasodilators. Am J Med Sci. 1987;294(4):238-243. doi:10.1097/00000441-198710000-00004
8. Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140. Published 2018 Jun 18. doi:10.1177/1758835918780140
9. Hansen RM, Ryan L, Anderson T, et al. Phase III study of bolus versus infusion fluorouracil with or without cisplatin in advanced colorectal cancer. J Natl Cancer Inst. 1996;88(10):668-674. doi:10.1093/jnci/88.10.668
10. Kim SM, Kwak CH, Lee B, et al. A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med. 2012;27(3):342-345. doi:10.3904/kjim.2012.27.3.342
11. Swarup S, Patibandla S, Grossman SA. Coronary Artery Vasospasm. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2021.
12. Beijk MA, Vlastra WV, Delewi R, et al. Myocardial infarction with non-obstructive coronary arteries: a focus on vasospastic angina. Neth Heart J. 2019;27(5):237-245. doi:10.1007/s12471-019-1232-7
13. Giza DE, Boccalandro F, Lopez-Mattei J, et al. Ischemic heart disease: special considerations in cardio-oncology. Curr Treat Options Cardiovasc Med. 2017;19(5):37. doi:10.1007/s11936-017-0535-5
14. Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont’s regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. 2005;35(5):265-270. doi:10.1093/jjco/hyi071
15. Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37(4):300-311. doi:10.1016/j.ctrv.2010.11.001
16. Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7(4):509-514. doi:10.1200/JCO.1989.7.4.509
17. Akpek G, Hartshorn KL. Failure of oral nitrate and calcium channel blocker therapy to prevent 5-fluorouracil-related myocardial ischemia: a case report. Cancer Chemother Pharmacol. 1999;43(2):157-161. doi:10.1007/s002800050877
A 40-year-old man with stage IV gastric adenocarcinoma was found to have coronary artery vasospasm in the setting of recent 5-fluorouracil administration.
A 40-year-old man with stage IV gastric adenocarcinoma was found to have coronary artery vasospasm in the setting of recent 5-fluorouracil administration.
Coronary artery vasospasm is a rare but well-known adverse effect of 5-fluorouracil (5-FU) that can be life threatening if unrecognized. Patients typically present with anginal chest pain and ST elevations on electrocardiogram (ECG) without atherosclerotic disease on coronary angiography. This phenomenon typically occurs during or shortly after infusion and resolves within hours to days after cessation of 5-FU.
In this report, we present an unusual case of coronary artery vasospasm that intermittently recurred for 25 days following 5-FU treatment in a 40-year-old male with stage IV gastric adenocarcinoma. We also review the literature on typical presentation and risk factors for 5-FU-induced coronary vasospasm, findings on coronary angiography, and management options.
5-FU is an IV administered antimetabolite chemotherapy commonly used to treat solid tumors, including gastrointestinal, pancreatic, breast, and head and neck tumors. 5-FU inhibits thymidylate synthase, which reduces levels of thymidine, a key pyrimidine nucleoside required for DNA replication within tumor cells.1 For several decades, 5-FU has remained one of the first-line drugs for colorectal cancer because it may be curative. It is the third most commonly used chemotherapy in the world and is included on the World Health Organization’s list of essential medicines.2
Cardiotoxicity occurs in 1.2 to 18% of patients who receive 5-FU therapy.3 Although there is variability in presentation for acute cardiotoxicity from 5-FU, including sudden death, angina pectoris, myocardial infarction, and ventricular arrhythmias, the mechanism most commonly implicated is coronary artery vasospasm.3 The direct observation of active coronary artery vasospasm during left heart catheterization is rare due its transient nature; however, several case studies have managed to demonstrate this.4,5 The pathophysiology of 5-FU-induced cardiotoxicity is unknown, but adverse effects on cardiac microvasculature, myocyte metabolism, platelet aggregation, and coronary vasoconstriction have all been proposed.3,6In the current case, we present a patient with stage IV gastric adenocarcinoma who complained of chest pain during hospitalization and was found to have coronary artery vasospasm in the setting of recent 5-FU administration. Following coronary angiography that showed a lack of atherosclerotic disease, the patient continued to experience episodes of chest pain with ST elevations on ECG that recurred despite cessation of 5-FU and repeated administration of vasodilatory medications.
Case Presentation
A male aged 40 years was admitted to the hospital for abdominal pain, with initial imaging concerning for partial small bowel obstruction. His history included recently diagnosed stage IV gastric adenocarcinoma complicated by peritoneal carcinomatosis status post initiation of infusional FOLFOX-4 (5-FU, leucovorin, and oxaliplatin) 11 days prior. The patient was treated for small bowel obstruction. However, several days after admission, he developed nonpleuritic, substernal chest pain unrelated to exertion and unrelieved by rest. The patient reported no known risk factors, family history, or personal history of coronary artery disease. Baseline echocardiography and ECG performed several months prior showed normal left ventricular function without ischemic findings.
Physical examination at the time of chest pain revealed a heart rate of 140 beats/min. The remainder of his vital signs were within normal range. There were no murmurs, rubs, gallops, or additional heart sounds heard on cardiac auscultation. Chest pain was not reproducible to palpation or positional in nature. An ECG demonstrated dynamic inferolateral ST elevations with reciprocal changes in leads I and aVL (Figure 1). A bedside echocardiogram showed hypokinesis of the septal wall. Troponin-I returned below the detectable level.
The patient was taken for emergent coronary catheterization, which demonstrated patent epicardial coronary arteries without atherosclerosis, a left ventricular ejection fraction of 60%, and a right dominant heart (Figures 2 and 3). Ventriculogram showed normal wall motion. Repeat troponin-I several hours after catheterization was again below detectable levels.
Given the patient’s acute onset of chest pain and inferolateral ST elevations seen on ECG, the working diagnosis prior to coronary catherization was acute coronary syndrome. The differential diagnosis included other causes of life-threatening chest pain, including pulmonary embolism, pneumonia, aortic dissection, myopericarditis, pericardial effusion, cardiac tamponade, or coronary artery vasospasm. Computed tomography (CT) angiography of the chest was not consistent with pulmonary embolism or other acute cardiopulmonary process. Based on findings from coronary angiography and recent exposure to 5-FU, as well as resolution followed by recurrence of chest pain and ECG changes over weeks, the most likely diagnosis after coronary catheterization was coronary artery vasospasm.
Treatment
Following catheterization, the patient returned to the medical intensive care unit, where he continued to report intermittent episodes of chest pain with ST elevations. In the following days, he was started on isosorbide mononitrate 150 mg daily and amlodipine 10 mg daily. Although these vasodilatory agents reduced the frequency of his chest pain episodes, intermittent chest pain associated with ST elevations on ECG continued even with maximal doses of isosorbide mononitrate and amlodipine. Administration of sublingual nitroglycerin during chest pain episodes effectively relieved his chest pain. Given the severity and frequency of the patient’s chest pain, the oncology consult team recommended foregoing further chemotherapeutic treatment with 5-FU.
Outcome
Despite holding 5-FU throughout the patient’s hospitalization and treating the patient with antianginal mediations, frequent chest pain episodes associated with ST elevations continued to recur until 25 days after his last treatment with 5-FU (Figure 4). The patient eventually expired during this hospital stay due to cancer-related complications.
Discussion
Coronary artery vasospasm is a well-known complication of 5-FU that can be life threatening if unrecognized.6-8 As seen in our case, patients typically present with anginal chest pain relieved with nitrates and ST elevations on ECG in the absence of occlusive macrovascular disease on coronary angiography.
A unique aspect of 5-FU is its variability in dose and frequency of administration across chemotherapeutic regimens. Particularly, 5-FU can be administered in daily intravenous bolus doses or as a continuous infusion for a protracted length of time. The spectrum of toxicity from 5-FU differs depending on the dose and frequency of administration. Bolus administration of 5-FU, for example, is thought to be associated with a higher rate of myelosuppression, while infusional administration of 5-FU is thought to be associated with a higher rate of cardiotoxicity and a higher tumor response rate.9
Most cases of coronary vasospasm occur either during infusion of 5-FU or within hours to days after completion. The median time of presentation for 5-FU-induced coronary artery vasospasm is about 12 hours postinfusion, while the most delayed presentation reported in the literature is 72 hours postinfusion.6,8 Delayed presentation of vasospasm may result from the release of potent vasoactive metabolites of 5-FU that accumulate over time; therefore, infusional administration may accentuate this effect.6,9 Remarkably, our patient’s chest pain episodes persisted for 25 days despite treatment with anti-anginal medications, highlighting the extent to which infusional 5-FU can produce a delay in adverse cardiotoxic effects and the importance of ongoing clinical vigilance after 5-FU exposure.
Vasospasm alone does not completely explain the spectrum of cardiac toxicity attributed to 5-FU administration. As in our case, coronary angiography during symptomatic episodes often fails to demonstrate coronary vasospasm.8 Additionally, ergonovine, an alkaloid agent used to assess coronary vasomotor function, failed to induce coronary vasospasm in some patients with suspected 5-FU-induced cardiac toxicity.10 The lack of vasospasm in some patients with 5-FU-induced cardiac toxicity suggests multiple independent effects of 5-FU on cardiac tissue that are poorly understood.
In the absence of obvious macrovascular effects, there also may be a deleterious effect of 5-FU on the coronary microvasculature that may result in coronary artery vasospasm. Though coronary microvasculature cannot be directly visualized, observation of slowed coronary blood velocity indicates a reduction in microvascular flow.8 Thus, the failure to observe epicardial coronary vasospasm in our patient does not preclude a vasospastic pathology.
The heterogeneous presentation of coronary artery vasospasm demands consideration of other disease processes such as atherosclerotic coronary artery disease, pericarditis, myopericarditis, primary arrythmias, and stress-induced cardiomyopathy, all of which have been described in association with 5-FU administration.8 A 12-lead ECG should be performed during a suspected attack. An ECG will typically demonstrate ST elevations corresponding to spasm of the involved vessel. Reciprocal ST depressions in the contralateral leads also may be seen. ECG may be useful in the acute setting to identify regional wall motion abnormalities or to rule out pericardial effusion as a cause. Cardiac biomarkers such as troponin-I, -C, and creatine kinase typically are less useful because they are often normal, even in known coronary artery vasospasm.11
Coronary angiography during an episode may show a localized region of vasospasm in an epicardial artery. Diffuse multivessel vasospasm does occur, and the location of vasospasm may change, but these events are rare. Under normal circumstances, provocative testing involving angiography with administration of acetylcholine, ergot agents, or hyperventilation can be performed. However, this type of investigation should be limited to specialized centers and should not be performed in the acute phase of the disease.12
Treatment of suspected coronary vasospasm in patients receiving 5-FU involves stopping the infusion and administering calcium channel blockers or oral nitrates to relieve anginal symptoms.13 5-FU-induced coronary artery vasospasm has a 90% rate of recurrence with subsequent infusions.8 If possible, alternate chemotherapy regimens should be considered once coronary artery vasospasm has been identified.14,15 If further 5-FU use is required, or if benefits are deemed to outweigh risks, infusions should be given in an inpatient setting with continuous cardiac monitoring.16
Calcium channel blockers and oral nitrates have been found to produce benefit in patients in acute settings; however, there is little evidence to attest to their effectiveness as prophylactic agents in those receiving 5-FU. Some reports demonstrate episodes where both calcium channel blockers and oral nitrates failed to prevent subsequent vasospasms.17 Although this was the case for our patient, short-acting sublingual nitroglycerin seemed to be effective in reducing the frequency of anginal symptoms.
Long-term outcomes have not been well investigated for patients with 5-FU-induced coronary vasospasm. However, many case reports show improvements in left ventricular function between 8 and 15 days after discontinuation of 5-FU.7,10 Although this would be a valuable topic for further research, the rarity of this phenomenon creates limitations.
Conclusions
5-FU is a first-line chemotherapy for gastrointestinal cancers that is generally well tolerated but may be associated with potentially life-threatening cardiotoxic effects, of which coronary artery vasospasm is the most common. Coronary artery vasospasm presents with anginal chest pain and ST elevations on ECG that can be indistinguishable from acute coronary syndrome. Diagnosis requires cardiac catheterization, which will reveal patent coronary arteries. Infusional administration of 5-FU may be more likely to produce late cardiotoxic effects and a longer period of persistent symptoms, necessitating close monitoring for days or even weeks from last administration of 5-FU. Coronary artery vasospasm should be treated with anti-anginal medications, though varying degrees of effectiveness can be seen; clinicians should remain vigilant for recurrent episodes of chest pain despite treatment.
Coronary artery vasospasm is a rare but well-known adverse effect of 5-fluorouracil (5-FU) that can be life threatening if unrecognized. Patients typically present with anginal chest pain and ST elevations on electrocardiogram (ECG) without atherosclerotic disease on coronary angiography. This phenomenon typically occurs during or shortly after infusion and resolves within hours to days after cessation of 5-FU.
In this report, we present an unusual case of coronary artery vasospasm that intermittently recurred for 25 days following 5-FU treatment in a 40-year-old male with stage IV gastric adenocarcinoma. We also review the literature on typical presentation and risk factors for 5-FU-induced coronary vasospasm, findings on coronary angiography, and management options.
5-FU is an IV administered antimetabolite chemotherapy commonly used to treat solid tumors, including gastrointestinal, pancreatic, breast, and head and neck tumors. 5-FU inhibits thymidylate synthase, which reduces levels of thymidine, a key pyrimidine nucleoside required for DNA replication within tumor cells.1 For several decades, 5-FU has remained one of the first-line drugs for colorectal cancer because it may be curative. It is the third most commonly used chemotherapy in the world and is included on the World Health Organization’s list of essential medicines.2
Cardiotoxicity occurs in 1.2 to 18% of patients who receive 5-FU therapy.3 Although there is variability in presentation for acute cardiotoxicity from 5-FU, including sudden death, angina pectoris, myocardial infarction, and ventricular arrhythmias, the mechanism most commonly implicated is coronary artery vasospasm.3 The direct observation of active coronary artery vasospasm during left heart catheterization is rare due its transient nature; however, several case studies have managed to demonstrate this.4,5 The pathophysiology of 5-FU-induced cardiotoxicity is unknown, but adverse effects on cardiac microvasculature, myocyte metabolism, platelet aggregation, and coronary vasoconstriction have all been proposed.3,6In the current case, we present a patient with stage IV gastric adenocarcinoma who complained of chest pain during hospitalization and was found to have coronary artery vasospasm in the setting of recent 5-FU administration. Following coronary angiography that showed a lack of atherosclerotic disease, the patient continued to experience episodes of chest pain with ST elevations on ECG that recurred despite cessation of 5-FU and repeated administration of vasodilatory medications.
Case Presentation
A male aged 40 years was admitted to the hospital for abdominal pain, with initial imaging concerning for partial small bowel obstruction. His history included recently diagnosed stage IV gastric adenocarcinoma complicated by peritoneal carcinomatosis status post initiation of infusional FOLFOX-4 (5-FU, leucovorin, and oxaliplatin) 11 days prior. The patient was treated for small bowel obstruction. However, several days after admission, he developed nonpleuritic, substernal chest pain unrelated to exertion and unrelieved by rest. The patient reported no known risk factors, family history, or personal history of coronary artery disease. Baseline echocardiography and ECG performed several months prior showed normal left ventricular function without ischemic findings.
Physical examination at the time of chest pain revealed a heart rate of 140 beats/min. The remainder of his vital signs were within normal range. There were no murmurs, rubs, gallops, or additional heart sounds heard on cardiac auscultation. Chest pain was not reproducible to palpation or positional in nature. An ECG demonstrated dynamic inferolateral ST elevations with reciprocal changes in leads I and aVL (Figure 1). A bedside echocardiogram showed hypokinesis of the septal wall. Troponin-I returned below the detectable level.
The patient was taken for emergent coronary catheterization, which demonstrated patent epicardial coronary arteries without atherosclerosis, a left ventricular ejection fraction of 60%, and a right dominant heart (Figures 2 and 3). Ventriculogram showed normal wall motion. Repeat troponin-I several hours after catheterization was again below detectable levels.
Given the patient’s acute onset of chest pain and inferolateral ST elevations seen on ECG, the working diagnosis prior to coronary catherization was acute coronary syndrome. The differential diagnosis included other causes of life-threatening chest pain, including pulmonary embolism, pneumonia, aortic dissection, myopericarditis, pericardial effusion, cardiac tamponade, or coronary artery vasospasm. Computed tomography (CT) angiography of the chest was not consistent with pulmonary embolism or other acute cardiopulmonary process. Based on findings from coronary angiography and recent exposure to 5-FU, as well as resolution followed by recurrence of chest pain and ECG changes over weeks, the most likely diagnosis after coronary catheterization was coronary artery vasospasm.
Treatment
Following catheterization, the patient returned to the medical intensive care unit, where he continued to report intermittent episodes of chest pain with ST elevations. In the following days, he was started on isosorbide mononitrate 150 mg daily and amlodipine 10 mg daily. Although these vasodilatory agents reduced the frequency of his chest pain episodes, intermittent chest pain associated with ST elevations on ECG continued even with maximal doses of isosorbide mononitrate and amlodipine. Administration of sublingual nitroglycerin during chest pain episodes effectively relieved his chest pain. Given the severity and frequency of the patient’s chest pain, the oncology consult team recommended foregoing further chemotherapeutic treatment with 5-FU.
Outcome
Despite holding 5-FU throughout the patient’s hospitalization and treating the patient with antianginal mediations, frequent chest pain episodes associated with ST elevations continued to recur until 25 days after his last treatment with 5-FU (Figure 4). The patient eventually expired during this hospital stay due to cancer-related complications.
Discussion
Coronary artery vasospasm is a well-known complication of 5-FU that can be life threatening if unrecognized.6-8 As seen in our case, patients typically present with anginal chest pain relieved with nitrates and ST elevations on ECG in the absence of occlusive macrovascular disease on coronary angiography.
A unique aspect of 5-FU is its variability in dose and frequency of administration across chemotherapeutic regimens. Particularly, 5-FU can be administered in daily intravenous bolus doses or as a continuous infusion for a protracted length of time. The spectrum of toxicity from 5-FU differs depending on the dose and frequency of administration. Bolus administration of 5-FU, for example, is thought to be associated with a higher rate of myelosuppression, while infusional administration of 5-FU is thought to be associated with a higher rate of cardiotoxicity and a higher tumor response rate.9
Most cases of coronary vasospasm occur either during infusion of 5-FU or within hours to days after completion. The median time of presentation for 5-FU-induced coronary artery vasospasm is about 12 hours postinfusion, while the most delayed presentation reported in the literature is 72 hours postinfusion.6,8 Delayed presentation of vasospasm may result from the release of potent vasoactive metabolites of 5-FU that accumulate over time; therefore, infusional administration may accentuate this effect.6,9 Remarkably, our patient’s chest pain episodes persisted for 25 days despite treatment with anti-anginal medications, highlighting the extent to which infusional 5-FU can produce a delay in adverse cardiotoxic effects and the importance of ongoing clinical vigilance after 5-FU exposure.
Vasospasm alone does not completely explain the spectrum of cardiac toxicity attributed to 5-FU administration. As in our case, coronary angiography during symptomatic episodes often fails to demonstrate coronary vasospasm.8 Additionally, ergonovine, an alkaloid agent used to assess coronary vasomotor function, failed to induce coronary vasospasm in some patients with suspected 5-FU-induced cardiac toxicity.10 The lack of vasospasm in some patients with 5-FU-induced cardiac toxicity suggests multiple independent effects of 5-FU on cardiac tissue that are poorly understood.
In the absence of obvious macrovascular effects, there also may be a deleterious effect of 5-FU on the coronary microvasculature that may result in coronary artery vasospasm. Though coronary microvasculature cannot be directly visualized, observation of slowed coronary blood velocity indicates a reduction in microvascular flow.8 Thus, the failure to observe epicardial coronary vasospasm in our patient does not preclude a vasospastic pathology.
The heterogeneous presentation of coronary artery vasospasm demands consideration of other disease processes such as atherosclerotic coronary artery disease, pericarditis, myopericarditis, primary arrythmias, and stress-induced cardiomyopathy, all of which have been described in association with 5-FU administration.8 A 12-lead ECG should be performed during a suspected attack. An ECG will typically demonstrate ST elevations corresponding to spasm of the involved vessel. Reciprocal ST depressions in the contralateral leads also may be seen. ECG may be useful in the acute setting to identify regional wall motion abnormalities or to rule out pericardial effusion as a cause. Cardiac biomarkers such as troponin-I, -C, and creatine kinase typically are less useful because they are often normal, even in known coronary artery vasospasm.11
Coronary angiography during an episode may show a localized region of vasospasm in an epicardial artery. Diffuse multivessel vasospasm does occur, and the location of vasospasm may change, but these events are rare. Under normal circumstances, provocative testing involving angiography with administration of acetylcholine, ergot agents, or hyperventilation can be performed. However, this type of investigation should be limited to specialized centers and should not be performed in the acute phase of the disease.12
Treatment of suspected coronary vasospasm in patients receiving 5-FU involves stopping the infusion and administering calcium channel blockers or oral nitrates to relieve anginal symptoms.13 5-FU-induced coronary artery vasospasm has a 90% rate of recurrence with subsequent infusions.8 If possible, alternate chemotherapy regimens should be considered once coronary artery vasospasm has been identified.14,15 If further 5-FU use is required, or if benefits are deemed to outweigh risks, infusions should be given in an inpatient setting with continuous cardiac monitoring.16
Calcium channel blockers and oral nitrates have been found to produce benefit in patients in acute settings; however, there is little evidence to attest to their effectiveness as prophylactic agents in those receiving 5-FU. Some reports demonstrate episodes where both calcium channel blockers and oral nitrates failed to prevent subsequent vasospasms.17 Although this was the case for our patient, short-acting sublingual nitroglycerin seemed to be effective in reducing the frequency of anginal symptoms.
Long-term outcomes have not been well investigated for patients with 5-FU-induced coronary vasospasm. However, many case reports show improvements in left ventricular function between 8 and 15 days after discontinuation of 5-FU.7,10 Although this would be a valuable topic for further research, the rarity of this phenomenon creates limitations.
Conclusions
5-FU is a first-line chemotherapy for gastrointestinal cancers that is generally well tolerated but may be associated with potentially life-threatening cardiotoxic effects, of which coronary artery vasospasm is the most common. Coronary artery vasospasm presents with anginal chest pain and ST elevations on ECG that can be indistinguishable from acute coronary syndrome. Diagnosis requires cardiac catheterization, which will reveal patent coronary arteries. Infusional administration of 5-FU may be more likely to produce late cardiotoxic effects and a longer period of persistent symptoms, necessitating close monitoring for days or even weeks from last administration of 5-FU. Coronary artery vasospasm should be treated with anti-anginal medications, though varying degrees of effectiveness can be seen; clinicians should remain vigilant for recurrent episodes of chest pain despite treatment.
1. Wacker A, Lersch C, Scherpinski U, Reindl L, Seyfarth M. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology. 2003;65(2):108-112. doi:10.1159/000072334
2. World Health Organization Model List of Essential Medicines, 21st List, 2019. Accessed April 14, 2021. https://apps.who.int/iris/rest/bitstreams/1237479/retrieve
3. Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58(4):487-493. doi:10.1007/s00280-005-0178-1
4. Shoemaker LK, Arora U, Rocha Lima CM. 5-fluorouracil-induced coronary vasospasm. Cancer Control. 2004;11(1):46-49. doi:10.1177/107327480401100207
5. Luwaert RJ, Descamps O, Majois F, Chaudron JM, Beauduin M. Coronary artery spasm induced by 5-fluorouracil. Eur Heart J. 1991;12(3):468-470. doi:10.1093/oxfordjournals.eurheartj.a059919
6. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191-202. doi:10.1517/14740330902733961
7. Patel B, Kloner RA, Ensley J, Al-Sarraf M, Kish J, Wynne J. 5-Fluorouracil cardiotoxicity: left ventricular dysfunction and effect of coronary vasodilators. Am J Med Sci. 1987;294(4):238-243. doi:10.1097/00000441-198710000-00004
8. Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140. Published 2018 Jun 18. doi:10.1177/1758835918780140
9. Hansen RM, Ryan L, Anderson T, et al. Phase III study of bolus versus infusion fluorouracil with or without cisplatin in advanced colorectal cancer. J Natl Cancer Inst. 1996;88(10):668-674. doi:10.1093/jnci/88.10.668
10. Kim SM, Kwak CH, Lee B, et al. A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med. 2012;27(3):342-345. doi:10.3904/kjim.2012.27.3.342
11. Swarup S, Patibandla S, Grossman SA. Coronary Artery Vasospasm. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2021.
12. Beijk MA, Vlastra WV, Delewi R, et al. Myocardial infarction with non-obstructive coronary arteries: a focus on vasospastic angina. Neth Heart J. 2019;27(5):237-245. doi:10.1007/s12471-019-1232-7
13. Giza DE, Boccalandro F, Lopez-Mattei J, et al. Ischemic heart disease: special considerations in cardio-oncology. Curr Treat Options Cardiovasc Med. 2017;19(5):37. doi:10.1007/s11936-017-0535-5
14. Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont’s regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. 2005;35(5):265-270. doi:10.1093/jjco/hyi071
15. Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37(4):300-311. doi:10.1016/j.ctrv.2010.11.001
16. Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7(4):509-514. doi:10.1200/JCO.1989.7.4.509
17. Akpek G, Hartshorn KL. Failure of oral nitrate and calcium channel blocker therapy to prevent 5-fluorouracil-related myocardial ischemia: a case report. Cancer Chemother Pharmacol. 1999;43(2):157-161. doi:10.1007/s002800050877
1. Wacker A, Lersch C, Scherpinski U, Reindl L, Seyfarth M. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology. 2003;65(2):108-112. doi:10.1159/000072334
2. World Health Organization Model List of Essential Medicines, 21st List, 2019. Accessed April 14, 2021. https://apps.who.int/iris/rest/bitstreams/1237479/retrieve
3. Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58(4):487-493. doi:10.1007/s00280-005-0178-1
4. Shoemaker LK, Arora U, Rocha Lima CM. 5-fluorouracil-induced coronary vasospasm. Cancer Control. 2004;11(1):46-49. doi:10.1177/107327480401100207
5. Luwaert RJ, Descamps O, Majois F, Chaudron JM, Beauduin M. Coronary artery spasm induced by 5-fluorouracil. Eur Heart J. 1991;12(3):468-470. doi:10.1093/oxfordjournals.eurheartj.a059919
6. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191-202. doi:10.1517/14740330902733961
7. Patel B, Kloner RA, Ensley J, Al-Sarraf M, Kish J, Wynne J. 5-Fluorouracil cardiotoxicity: left ventricular dysfunction and effect of coronary vasodilators. Am J Med Sci. 1987;294(4):238-243. doi:10.1097/00000441-198710000-00004
8. Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140. Published 2018 Jun 18. doi:10.1177/1758835918780140
9. Hansen RM, Ryan L, Anderson T, et al. Phase III study of bolus versus infusion fluorouracil with or without cisplatin in advanced colorectal cancer. J Natl Cancer Inst. 1996;88(10):668-674. doi:10.1093/jnci/88.10.668
10. Kim SM, Kwak CH, Lee B, et al. A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med. 2012;27(3):342-345. doi:10.3904/kjim.2012.27.3.342
11. Swarup S, Patibandla S, Grossman SA. Coronary Artery Vasospasm. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2021.
12. Beijk MA, Vlastra WV, Delewi R, et al. Myocardial infarction with non-obstructive coronary arteries: a focus on vasospastic angina. Neth Heart J. 2019;27(5):237-245. doi:10.1007/s12471-019-1232-7
13. Giza DE, Boccalandro F, Lopez-Mattei J, et al. Ischemic heart disease: special considerations in cardio-oncology. Curr Treat Options Cardiovasc Med. 2017;19(5):37. doi:10.1007/s11936-017-0535-5
14. Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont’s regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. 2005;35(5):265-270. doi:10.1093/jjco/hyi071
15. Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37(4):300-311. doi:10.1016/j.ctrv.2010.11.001
16. Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7(4):509-514. doi:10.1200/JCO.1989.7.4.509
17. Akpek G, Hartshorn KL. Failure of oral nitrate and calcium channel blocker therapy to prevent 5-fluorouracil-related myocardial ischemia: a case report. Cancer Chemother Pharmacol. 1999;43(2):157-161. doi:10.1007/s002800050877
Beneath the Surface: Massive Retroperitoneal Liposarcoma Masquerading as Meralgia Paresthetica
In patients presenting with focal neurologic findings involving the lower extremities, a thorough abdominal examination should be considered an integral part of the full neurologic work up.
Meralgia paresthetica (MP) is a sensory mononeuropathy of the lateral femoral cutaneous nerve (LFCN), clinically characterized by numbness, pain, and paresthesias involving the anterolateral aspect of the thigh. Estimates of MP incidence are derived largely from observational studies and reported to be about 3.2 to 4.3 cases per 10,000 patient-years.1,2 Although typically arising during midlife and especially in the context of comorbid obesity, diabetes mellitus (DM), and excessive alcohol consumption, MP may occur at any age, and bears a slight predilection for males.2-4
MP may be divided etiologically into iatrogenic and spontaneous subtypes.5 Iatrogenic cases generally are attributable to nerve injury in the setting of direct or indirect trauma (such as with patient malpositioning) arising in the context of multiple forms of procedural or surgical intervention (Table). Spontaneous MP is primarily thought to occur as a result of LFCN compression at the level of the inguinal ligament, wherein internal or external pressures may promote LFCN entrapment and resultant functional disruption (Figure 1).6,7
External forces, such as tight garments, wallets, or even elements of modern body armor, have been reported to provoke MP.8-11 Alternatively, states of increased intraabdominal pressure, such as obesity, ascites, and pregnancy may predispose to LFCN compression.2,12,13 Less commonly, lumbar radiculopathy, pelvic masses, and several forms of retroperitoneal pathology may present with clinical symptomatology indistinguishable from MP.14-17 Importantly, many of these represent must-not-miss diagnoses, and may be suggested via a focused history and physical examination.
Here, we present a case of MP secondary to a massive retroperitoneal sarcoma, ultimately drawing renewed attention to the known association of MP and retroperitoneal pathology, and therein highlighting the utility of a dedicated review of systems to identify red-flag features in patients who present with MP and a thorough abdominal examination in all patients presenting with focal neurologic deficits involving the lower extremities.
Case Presentation
A male Vietnam War veteran aged 69 years presented to a primary care clinic at West Roxbury Veterans Affairs Medical Center (WRVAMC) in Massachusetts with progressive right lower extremity numbness. Three months prior to this visit, he was evaluated in an urgent care clinic at WRVAMC for 6 months of numbness and increasingly painful nocturnal paresthesias involving the same extremity. A targeted physical examination at that visit revealed an obese male wearing tight suspenders, as well as focally diminished sensation to light touch involving the anterolateral aspect of the thigh, extending from just below the right hip to above the knee. Sensation in the medial thigh was spared. Strength and reflexes were normal in the bilateral lower extremities. An abdominal examination was not performed. He received a diagnosis of MP and counseled regarding weight loss, glycemic control, garment optimization, and conservative analgesia with as-needed nonsteroidal anti-inflammatory drugs. He was instructed to follow-up closely with his primary care physician for further monitoring.
During the current visit, the patient reported 2 atraumatic falls the prior 2 months, attributed to escalating right leg weakness. The patient reported that ascending stairs had become difficult, and he was unable to cross his right leg over his left while in a seated position. The territory of numbness expanded to his front and inner thigh. Although previously he was able to hike 4 miles, he now was unable to walk more than half of a mile without developing shortness of breath. He reported frequent urination without hematuria and a recent weight gain of 8 pounds despite early satiety.
His medical history included hypertension, hypercholesterolemia, truncal obesity, noninsulin dependent DM, coronary artery disease, atrial flutter, transient ischemic attack, and benign positional paroxysmal vertigo. He was exposed to Agent Orange during his service in Vietnam. Family history was notable for breast cancer (mother), lung cancer (father), and an unspecified form of lymphoma (brother). He had smoked approximately 2 packs of cigarettes daily for 15 years but quit 38 years prior. He reported consuming on average 3 alcohol-containing drinks per week and no illicit drug use. He was adherent with all medications, including furosemide 40 mg daily, losartan 25 mg daily, metoprolol succinate 50 mg daily, atorvastatin 80 mg daily, metformin 500 mg twice daily, and rivaroxaban 20 mg daily with dinner.
His vital signs included a blood pressure of 123/58 mmHg, a pulse of 74 beats per minute, a respiratory rate of 16 breaths per minute, and an oxygen saturation of 94% on ambient air. His temperature was recorded at 96.7°F, and his weight was 234 pounds with a body mass index (BMI) of 34. He was well groomed and in no acute distress. His cardiopulmonary examination was normal. Carotid, radial, and bilateral dorsalis pedis pulsations were 2+ bilaterally, and no jugular venous distension was observed at 30°. The abdomen was protuberant. Nonshifting dullness to percussion and firmness to palpation was observed throughout right upper and lower quadrants, with hyperactive bowel sounds primarily localized to the left upper and lower quadrants.
Neurologic examination revealed symmetric facies with normal phonation and diction. He was spontaneously moving all extremities, and his gait was normal. Sensation to light touch was severely diminished throughout the anterolateral and medial thigh, extending to the level of the knee, and otherwise reduced in a stocking-type pattern over the bilateral feet and toes. His right hip flexion, adduction, as well as internal and external rotation were focally diminished to 4- out of 5. Right knee extension was 4+ out of 5. Strength was otherwise 5 out of 5. The patient exhibited asymmetric Patellar reflexes—absent on the right and 2+ on the left. Achilles reflexes were absent bilaterally. Straight-leg raise test was negative bilaterally and did not clearly exacerbate his right leg numbness or paresthesias. There were no notable fasciculations. There was 2+ bilateral lower extremity pitting edema appreciated to the level of the midshin (right greater than left), without palpable cords or new skin lesions.
Upon referral to the neurology service, the patient underwent electromyography, which revealed complex repetitive discharges in the right tibialis anterior and pattern of reduced recruitment upon activation of the right vastus medialis, collectively suggestive of an L3-4 plexopathy. The patient was admitted for expedited workup.
A complete blood count and metabolic panel that were taken in the emergency department were normal, save for a serum bicarbonate of 30 mEq/L. His hemoglobin A1c was 6.6%. Computed tomography (CT) of the abdomen and pelvis with IV contrast was obtained, and notable for a 30 cm fat-containing right-sided retroperitoneal mass with associated solid nodular components and calcification (Figure 2). No enhancement of the lesion was observed. There was significant associated mass effect, with superior displacement of the liver and right hemidiaphragm, as well as superomedial deflection of the right kidney, inferior vena cava, and other intraabdominal organs. Subsequent imaging with a CT of the chest, as well as magnetic resonance imaging of the brain, were without evidence of metastatic disease.
18Fluorodeoxyglucose-positron emission tomography (FDG-PET) was performed and demonstrated heterogeneous FDG avidity throughout the mass (SUVmax 5.9), as well as poor delineation of the boundary of the right psoas major, consistent with muscular invasion (Figure 3). The FDG-PET also revealed intense tracer uptake within the left prostate (SUVmax 26), concerning for a concomitant prostate malignancy.
To facilitate tissue diagnosis, the patient underwent a CT-guided biopsy of the retroperitoneal mass. Subsequent histopathologic analysis revealed a primarily well-differentiated spindle cell lesion with occasional adipocytic atypia, and a superimposed hypercellular element characterized by the presence of pleomorphic high-grade spindled cells. The neoplastic spindle cells were MDM2-positive by both immunohistochemistry and fluorescence in situ hybridization (FISH), and negative for pancytokeratin, smooth muscle myosin, and S100. The findings were collectively consistent with a dedifferentiated liposarcoma (DDLPS).
Given the focus of FDG avidity observed on the PET, the patient underwent a transrectal ultrasound-guided biopsy of the prostate, which yielded diagnosis of a concomitant high-risk (Gleason 4+4) prostate adenocarcinoma. A bone scan did not reveal evidence of osseous metastatic disease.
Outcome
The patient was treated with external beam radiotherapy (EBRT) delivered simultaneously to both the prostate and high-risk retroperitoneal margins of the DDLPS, as well as concurrent androgen deprivation therapy. Five months after completed radiotherapy, resection of the DDLPS was attempted. However, palliative tumor debulking was instead performed due to extensive locoregional invasion with involvement of the posterior peritoneum and ipsilateral quadratus, iliopsoas, and psoas muscles, as well as the adjacent lumbar nerve roots.
At present, the patient is undergoing surveillance imaging every 3 months to reevaluate his underlying disease burden, which has thus far been radiographically stable. Current management at the primary care level is focused on preserving quality of life, particularly maintaining mobility and functional independence.
Discussion
Although generally a benign entrapment neuropathy, MP bears well-established associations with multiple forms of must-not-miss pathology. Here, we present the case of a veteran in whom MP was the index presentation of a massive retroperitoneal liposarcoma, stressing the importance of a thorough history and physical examination in all patients presenting with MP. The case presented herein highlights many of the red-flag signs and symptoms that primary care physicians might encounter in patients with retroperitoneal pathology, including MP and MP-like syndromes (Figure 4).
In this case, the pretest probability of a spontaneous and uncomplicated MP was high given the patient’s sex, age, body habitus, and DM; however, there important atypia that emerged as the case evolved, including: (1) the progressive course; (2) proximal right lower extremity weakness; (3) asymmetric patellar reflexes; and (4) numerous clinical stigmata of intraabdominal mass effect. The patient exhibited abnormalities on abdominal examination that suggested the presence of an underlying intraabdominal mass, providing key diagnostic insight into this case. Given the slowly progressive nature of liposarcomas, we feel the abnormalities appreciated on abdominal examination were likely apparent during the initial presentation.18
There are numerous cognitive biases that may explain why an abdominal examination was not prioritized during the initial presentation. Namely, the patient’s numerous risk factors for spontaneous MP, as detailed above, may have contributed to framing bias that limited consideration of alternative diagnoses. In addition, the patient’s physical examination likely contributed to search satisfaction, whereby alternative diagnoses were not further entertained after discovery of findings consistent with spontaneous MP.19 Finally, it remains conceivable that an abdominal examination was not prioritized as it is often perceived as being distinct from, rather than an integral part of, the neurologic examination.20 Given that numerous neurologic disorders may present with abdominal pathology, we feel a thorough abdominal examination should be considered part of the full neurologic examination, especially in cases presenting with focal neurologic findings involving the lower extremities.21
Collectively, this case alludes to the importance of close clinical follow-up, as well as adequate anticipatory patient guidance in cases of suspected MP. In most patients, the clinical course of spontaneous MP is benign and favorable, with up to 85% of patients experiencing resolution within 4 to 6 months of the initial presentation.22 Common conservative measures include weight loss, garment optimization, and nonsteroidal anti-inflammatory drugs as needed for analgesia. In refractory cases, procedural interventions such as with neurolysis or resection of the lateral femoral cutaneous nerve, may be required after the ruling out of alternative diagnoses.23,24
Importantly, in even prolonged and resistant cases of MP, patient discomfort remains localized to the territory of the LFCN. Additional lower motor neuron signs, such as an expanding territory of sensory involvement, muscle weakness, or diminished reflexes, should prompt additional testing for alternative diagnoses. In addition, clinical findings concerning for intraabdominal mass effect, many of which were observed in this case, should lead to further evaluation and expeditious cross-sectional imaging. Although this patient’s early satiety, polyuria, bilateral lower extremity edema, weight gain, and lumbar plexopathy each may be explained by direct compression, invasion, or displacement, his report of progressive exertional dyspnea merits further discussion.
Exertional dyspnea is an uncommon complication of soft tissue sarcoma, reported almost exclusively in cases with cardiac, mediastinal, or other thoracic involvement.25-28 In this case, there was no evidence of thoracic involvement, either through direct extension or metastasis. Instead, the patient’s exertional dyspnea may have been attributable to increased intraabdominal pressure leading to compromised diaphragm excursion and reduced pulmonary reserve. In addition, the radiographic findings also raise the possibility of a potential contribution from preload failure due to IVC compression. Overall, dyspnea is a concerning feature that may suggest advanced disease.
Despite the value of a thorough history and physical examination in patients with MP, major clinical guidelines from neurologic, neurosurgical, and orthopedic organizations do not formally address MP evaluation and management. Further, proposed clinical practice algorithms are inconsistent in their recommendations regarding the identification of red-flag features and ruling out of alternative diagnoses.22,29,30 To supplement the abdominal examination, it would be reasonable to perform a pelvic compression test (PCT) in patients presenting with suspected MP. The PCT is a highly sensitive and specific provocative maneuver shown to enable reliable differentiation between MP and lumbar radiculopathy, and is performed by placing downward force on the anterior superior iliac spine of the affected extremity for 45 seconds with the patient in the lateral recumbent position.31 As this maneuver is intended to force relaxation of the inguinal ligament, thereby relieving pressure on the LFCN, improvement in the patient’s symptoms with the PCT is consistent with MP.
Conclusions
1. van Slobbe AM, Bohnen AM, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol. 2004;251(3):294-297. doi:10.1007/s00415-004-0310-x
2. Parisi TJ, Mandrekar J, Dyck PJ, Klein CJ. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology. 2011;77(16):1538-1542. doi:10.1212/WNL.0b013e318233b356
3. Ecker AD. Diagnosis of meralgia paresthetica. JAMA. 1985;253(7):976.
4. Massey EW, Pellock JM. Meralgia paraesthetica in a child. J Pediatr. 1978;93(2):325-326. doi:10.1016/s0022-3476(78)80566-6
5. Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8(8):669-677. doi:10.1111/j.1526-4637.2006.00227.x
6. Berini SE, Spinner RJ, Jentoft ME, et al. Chronic meralgia paresthetica and neurectomy: a clinical pathologic study. Neurology. 2014;82(17):1551-1555. doi:10.1212/WNL.0000000000000367
7. Payne RA, Harbaugh K, Specht CS, Rizk E. Correlation of histopathology and clinical symptoms in meralgia paresthetica. Cureus. 2017;9(10):e1789. Published 2017 Oct 20. doi:10.7759/cureus.1789
8. Boyce JR. Meralgia paresthetica and tight trousers. JAMA. 1984;251(12):1553.
9. Orton D. Meralgia paresthetica from a wallet. JAMA. 1984;252(24):3368.
10. Fargo MV, Konitzer LN. Meralgia paresthetica due to body armor wear in U.S. soldiers serving in Iraq: a case report and review of the literature. Mil Med. 2007;172(6):663-665. doi:10.7205/milmed.172.6.663
11. Korkmaz N, Ozçakar L. Meralgia paresthetica in a policeman: the belt or the gun. Plast Reconstr Surg. 2004;114(4):1012-1013. doi:10.1097/01.prs.0000138706.86633.01
12. Gooding MS, Evangelista V, Pereira L. Carpal Tunnel Syndrome and Meralgia Paresthetica in Pregnancy. Obstet Gynecol Surv. 2020;75(2):121-126. doi:10.1097/OGX.0000000000000745
13. Pauwels A, Amarenco P, Chazouillères O, Pigot F, Calmus Y, Lévy VG. Une complication rare et méconnue de l’ascite: la méralgie paresthésique [Unusual and unknown complication of ascites: meralgia paresthetica]. Gastroenterol Clin Biol. 1990;14(3):295.
14. Braddom RL. L2 rather than L1 radiculopathy mimics meralgia paresthetica. Muscle Nerve. 2010;42(5):842. doi:10.1002/mus.21826
15. Suber DA, Massey EW. Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol. 1979;53(2):257-258.
16. Flowers RS. Meralgia paresthetica. A clue to retroperitoneal malignant tumor. Am J Surg. 1968;116(1):89-92. doi:10.1016/0002-9610(68)90423-6
17. Yi TI, Yoon TH, Kim JS, Lee GE, Kim BR. Femoral neuropathy and meralgia paresthetica secondary to an iliacus hematoma. Ann Rehabil Med. 2012;36(2):273-277. doi:10.5535/arm.2012.36.2.273
18. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;36(2):151-159. doi:10.1200/JCO.2017.74.9598
19. O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48(3):225-232. doi:10.4997/JRCPE.2018.306
20. Bickley, LS. Bates’ Guide to Physical Examination and History Taking. 12th Edition. Wolters Kluwer Health/Lippincott Williams and Wilkins; 2016.
21. Bhavsar AS, Verma S, Lamba R, Lall CG, Koenigsknecht V, Rajesh A. Abdominal manifestations of neurologic disorders. Radiographics. 2013;33(1):135-153. doi:10.1148/rg.331125097
22. Dureja GP, Gulaya V, Jayalakshmi TS, Mandal P. Management of meralgia paresthetica: a multimodality regimen. Anesth Analg. 1995;80(5):1060-1061. doi:10.1097/00000539-199505000-00043
23. Patijn J, Mekhail N, Hayek S, Lataster A, van Kleef M, Van Zundert J. Meralgia paresthetica. Pain Pract. 2011;11(3):302-308. doi:10.1111/j.1533-2500.2011.00458.x24. Ivins GK. Meralgia paresthetica, the elusive diagnosis: clinical experience with 14 adult patients. Ann Surg. 2000;232(2):281-286. doi:10.1097/00000658-200008000-00019
25. Munin MA, Goerner MS, Raggio I, et al. A rare cause of dyspnea: undifferentiated pleomorphic sarcoma in the left atrium. Cardiol Res. 2017;8(5):241-245. doi:10.14740/cr590w
26. Nguyen A, Awad WI. Cardiac sarcoma arising from malignant transformation of a preexisting atrial myxoma. Ann Thorac Surg. 2016;101(4):1571-1573. doi:10.1016/j.athoracsur.2015.05.129
27. Jiang S, Li J, Zeng Q, Liang J. Pulmonary artery intimal sarcoma misdiagnosed as pulmonary embolism: a case report. Oncol Lett. 2017;13(4):2713-2716. doi:10.3892/ol.2017.5775
28. Cojocaru A, Oliveira PJ, Pellecchia C. A pleural presentation of a rare soft tissue sarcoma. Am J Resp Crit Care Med. 2012;185:A5201. doi:10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5201
29. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(5):336-344. doi:10.5435/00124635-200109000-00007
30. Cheatham SW, Kolber MJ, Salamh PA. Meralgia paresthetica: a review of the literature. Int J Sports Phys Ther. 2013;8(6):883-893.
31. Nouraei SA, Anand B, Spink G, O’Neill KS. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60(4):696-700. doi:10.1227/01.NEU.0000255392.69914.F7
32. Antunes PE, Antunes MJ. Meralgia paresthetica after aortic valve surgery. J Heart Valve Dis. 1997;6(6):589-590.
33. Reddy YM, Singh D, Chikkam V, et al. Postprocedural neuropathy after atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36(3):279-285. doi:10.1007/s10840-012-9724-z
34. Butler R, Webster MW. Meralgia paresthetica: an unusual complication of cardiac catheterization via the femoral artery. Catheter Cardiovasc Interv. 2002;56(1):69-71. doi:10.1002/ccd.10149
35. Jellish WS, Oftadeh M. Peripheral nerve injury in cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):495-511. doi:10.1053/j.jvca.2017.08.030
36. Parsonnet V, Karasakalides A, Gielchinsky I, Hochberg M, Hussain SM. Meralgia paresthetica after coronary bypass surgery. J Thorac Cardiovasc Surg. 1991;101(2):219-221.
37. Macgregor AM, Thoburn EK. Meralgia paresthetica following bariatric surgery. Obes Surg. 1999;9(4):364-368. doi:10.1381/096089299765552945
38. Grace DM. Meralgia paresthetica after gastroplasty for morbid obesity. Can J Surg. 1987;30(1):64-65.
39. Polidori L, Magarelli M, Tramutoli R. Meralgia paresthetica as a complication of laparoscopic appendectomy. Surg Endosc. 2003;17(5):832. doi:10.1007/s00464-002-4279-1
40. Yamout B, Tayyim A, Farhat W. Meralgia paresthetica as a complication of laparoscopic cholecystectomy. Clin Neurol Neurosurg. 1994;96(2):143-144. doi:10.1016/0303-8467(94)90048-5
41. Broin EO, Horner C, Mealy K, et al. Meralgia paraesthetica following laparoscopic inguinal hernia repair. an anatomical analysis. Surg Endosc. 1995;9(1):76-78. doi:10.1007/BF00187893
42. Eubanks S, Newman L 3rd, Goehring L, et al. Meralgia paresthetica: a complication of laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3(5):381-385.
43. Atamaz F, Hepgüler S, Karasu Z, Kilic M. Meralgia paresthetica after liver transplantation: a case report. Transplant Proc. 2005;37(10):4424-4425. doi:10.1016/j.transproceed.2005.11.047
44. Chung KH, Lee JY, Ko TK, et al. Meralgia paresthetica affecting parturient women who underwent cesarean section -a case report-. Korean J Anesthesiol. 2010;59 Suppl(Suppl):S86-S89. doi:10.4097/kjae.2010.59.S.S86
45. Hutchins FL Jr, Huggins J, Delaney ML. Laparoscopic myomectomy-an unusual cause of meralgia paresthetica. J Am Assoc Gynecol Laparosc. 1998;5(3):309-311. doi:10.1016/s1074-3804(98)80039-x
46. Jones CD, Guiot L, Portelli M, Bullen T, Skaife P. Two interesting cases of meralgia paraesthetica. Pain Physician. 2017;20(6):E987-E989.
47. Peters G, Larner AJ. Meralgia paresthetica following gynecologic and obstetric surgery. Int J Gynaecol Obstet. 2006;95(1):42-43. doi:10.1016/j.ijgo.2006.05.025
48. Kvarnström N, Järvholm S, Johannesson L, Dahm-Kähler P, Olausson M, Brännström M. Live donors of the initial observational study of uterus transplantation-psychological and medical follow-up until 1 year after surgery in the 9 cases. Transplantation. 2017;101(3):664-670. doi:10.1097/TP.0000000000001567
49. Goulding K, Beaulé PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468(9):2397-2404. doi:10.1007/s11999-010-1406-5
50. Yamamoto T, Nagira K, Kurosaka M. Meralgia paresthetica occurring 40 years after iliac bone graft harvesting: case report. Neurosurgery. 2001;49(6):1455-1457. doi:10.1097/00006123-200112000-00028
51. Roqueplan F, Porcher R, Hamzé B, et al. Long-term results of percutaneous resection and interstitial laser ablation of osteoid osteomas. Eur Radiol. 2010;20(1):209-217. doi:10.1007/s00330-009-1537-9
52. Gupta A, Muzumdar D, Ramani PS. Meralgia paraesthetica following lumbar spine surgery: a study in 110 consecutive surgically treated cases. Neurol India. 2004;52(1):64-66.
53. Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine (Phila Pa 1976). 2005;30(18):E547-E550. doi:10.1097/01.brs.0000178821.14102.9d
54. Tejwani SG, Scaduto AA, Bowen RE. Transient meralgia paresthetica after pediatric posterior spine fusion. J Pediatr Orthop. 2006;26(4):530-533. doi:10.1097/01.bpo.0000217721.95480.9e
55. Peker S, Ay B, Sun I, Ozgen S, Pamir M. Meralgia paraesthetica: complications of prone position during lumbar disc surgery. Internet J Anesthesiol. 2003;8(1):24-29.
In patients presenting with focal neurologic findings involving the lower extremities, a thorough abdominal examination should be considered an integral part of the full neurologic work up.
In patients presenting with focal neurologic findings involving the lower extremities, a thorough abdominal examination should be considered an integral part of the full neurologic work up.
Meralgia paresthetica (MP) is a sensory mononeuropathy of the lateral femoral cutaneous nerve (LFCN), clinically characterized by numbness, pain, and paresthesias involving the anterolateral aspect of the thigh. Estimates of MP incidence are derived largely from observational studies and reported to be about 3.2 to 4.3 cases per 10,000 patient-years.1,2 Although typically arising during midlife and especially in the context of comorbid obesity, diabetes mellitus (DM), and excessive alcohol consumption, MP may occur at any age, and bears a slight predilection for males.2-4
MP may be divided etiologically into iatrogenic and spontaneous subtypes.5 Iatrogenic cases generally are attributable to nerve injury in the setting of direct or indirect trauma (such as with patient malpositioning) arising in the context of multiple forms of procedural or surgical intervention (Table). Spontaneous MP is primarily thought to occur as a result of LFCN compression at the level of the inguinal ligament, wherein internal or external pressures may promote LFCN entrapment and resultant functional disruption (Figure 1).6,7
External forces, such as tight garments, wallets, or even elements of modern body armor, have been reported to provoke MP.8-11 Alternatively, states of increased intraabdominal pressure, such as obesity, ascites, and pregnancy may predispose to LFCN compression.2,12,13 Less commonly, lumbar radiculopathy, pelvic masses, and several forms of retroperitoneal pathology may present with clinical symptomatology indistinguishable from MP.14-17 Importantly, many of these represent must-not-miss diagnoses, and may be suggested via a focused history and physical examination.
Here, we present a case of MP secondary to a massive retroperitoneal sarcoma, ultimately drawing renewed attention to the known association of MP and retroperitoneal pathology, and therein highlighting the utility of a dedicated review of systems to identify red-flag features in patients who present with MP and a thorough abdominal examination in all patients presenting with focal neurologic deficits involving the lower extremities.
Case Presentation
A male Vietnam War veteran aged 69 years presented to a primary care clinic at West Roxbury Veterans Affairs Medical Center (WRVAMC) in Massachusetts with progressive right lower extremity numbness. Three months prior to this visit, he was evaluated in an urgent care clinic at WRVAMC for 6 months of numbness and increasingly painful nocturnal paresthesias involving the same extremity. A targeted physical examination at that visit revealed an obese male wearing tight suspenders, as well as focally diminished sensation to light touch involving the anterolateral aspect of the thigh, extending from just below the right hip to above the knee. Sensation in the medial thigh was spared. Strength and reflexes were normal in the bilateral lower extremities. An abdominal examination was not performed. He received a diagnosis of MP and counseled regarding weight loss, glycemic control, garment optimization, and conservative analgesia with as-needed nonsteroidal anti-inflammatory drugs. He was instructed to follow-up closely with his primary care physician for further monitoring.
During the current visit, the patient reported 2 atraumatic falls the prior 2 months, attributed to escalating right leg weakness. The patient reported that ascending stairs had become difficult, and he was unable to cross his right leg over his left while in a seated position. The territory of numbness expanded to his front and inner thigh. Although previously he was able to hike 4 miles, he now was unable to walk more than half of a mile without developing shortness of breath. He reported frequent urination without hematuria and a recent weight gain of 8 pounds despite early satiety.
His medical history included hypertension, hypercholesterolemia, truncal obesity, noninsulin dependent DM, coronary artery disease, atrial flutter, transient ischemic attack, and benign positional paroxysmal vertigo. He was exposed to Agent Orange during his service in Vietnam. Family history was notable for breast cancer (mother), lung cancer (father), and an unspecified form of lymphoma (brother). He had smoked approximately 2 packs of cigarettes daily for 15 years but quit 38 years prior. He reported consuming on average 3 alcohol-containing drinks per week and no illicit drug use. He was adherent with all medications, including furosemide 40 mg daily, losartan 25 mg daily, metoprolol succinate 50 mg daily, atorvastatin 80 mg daily, metformin 500 mg twice daily, and rivaroxaban 20 mg daily with dinner.
His vital signs included a blood pressure of 123/58 mmHg, a pulse of 74 beats per minute, a respiratory rate of 16 breaths per minute, and an oxygen saturation of 94% on ambient air. His temperature was recorded at 96.7°F, and his weight was 234 pounds with a body mass index (BMI) of 34. He was well groomed and in no acute distress. His cardiopulmonary examination was normal. Carotid, radial, and bilateral dorsalis pedis pulsations were 2+ bilaterally, and no jugular venous distension was observed at 30°. The abdomen was protuberant. Nonshifting dullness to percussion and firmness to palpation was observed throughout right upper and lower quadrants, with hyperactive bowel sounds primarily localized to the left upper and lower quadrants.
Neurologic examination revealed symmetric facies with normal phonation and diction. He was spontaneously moving all extremities, and his gait was normal. Sensation to light touch was severely diminished throughout the anterolateral and medial thigh, extending to the level of the knee, and otherwise reduced in a stocking-type pattern over the bilateral feet and toes. His right hip flexion, adduction, as well as internal and external rotation were focally diminished to 4- out of 5. Right knee extension was 4+ out of 5. Strength was otherwise 5 out of 5. The patient exhibited asymmetric Patellar reflexes—absent on the right and 2+ on the left. Achilles reflexes were absent bilaterally. Straight-leg raise test was negative bilaterally and did not clearly exacerbate his right leg numbness or paresthesias. There were no notable fasciculations. There was 2+ bilateral lower extremity pitting edema appreciated to the level of the midshin (right greater than left), without palpable cords or new skin lesions.
Upon referral to the neurology service, the patient underwent electromyography, which revealed complex repetitive discharges in the right tibialis anterior and pattern of reduced recruitment upon activation of the right vastus medialis, collectively suggestive of an L3-4 plexopathy. The patient was admitted for expedited workup.
A complete blood count and metabolic panel that were taken in the emergency department were normal, save for a serum bicarbonate of 30 mEq/L. His hemoglobin A1c was 6.6%. Computed tomography (CT) of the abdomen and pelvis with IV contrast was obtained, and notable for a 30 cm fat-containing right-sided retroperitoneal mass with associated solid nodular components and calcification (Figure 2). No enhancement of the lesion was observed. There was significant associated mass effect, with superior displacement of the liver and right hemidiaphragm, as well as superomedial deflection of the right kidney, inferior vena cava, and other intraabdominal organs. Subsequent imaging with a CT of the chest, as well as magnetic resonance imaging of the brain, were without evidence of metastatic disease.
18Fluorodeoxyglucose-positron emission tomography (FDG-PET) was performed and demonstrated heterogeneous FDG avidity throughout the mass (SUVmax 5.9), as well as poor delineation of the boundary of the right psoas major, consistent with muscular invasion (Figure 3). The FDG-PET also revealed intense tracer uptake within the left prostate (SUVmax 26), concerning for a concomitant prostate malignancy.
To facilitate tissue diagnosis, the patient underwent a CT-guided biopsy of the retroperitoneal mass. Subsequent histopathologic analysis revealed a primarily well-differentiated spindle cell lesion with occasional adipocytic atypia, and a superimposed hypercellular element characterized by the presence of pleomorphic high-grade spindled cells. The neoplastic spindle cells were MDM2-positive by both immunohistochemistry and fluorescence in situ hybridization (FISH), and negative for pancytokeratin, smooth muscle myosin, and S100. The findings were collectively consistent with a dedifferentiated liposarcoma (DDLPS).
Given the focus of FDG avidity observed on the PET, the patient underwent a transrectal ultrasound-guided biopsy of the prostate, which yielded diagnosis of a concomitant high-risk (Gleason 4+4) prostate adenocarcinoma. A bone scan did not reveal evidence of osseous metastatic disease.
Outcome
The patient was treated with external beam radiotherapy (EBRT) delivered simultaneously to both the prostate and high-risk retroperitoneal margins of the DDLPS, as well as concurrent androgen deprivation therapy. Five months after completed radiotherapy, resection of the DDLPS was attempted. However, palliative tumor debulking was instead performed due to extensive locoregional invasion with involvement of the posterior peritoneum and ipsilateral quadratus, iliopsoas, and psoas muscles, as well as the adjacent lumbar nerve roots.
At present, the patient is undergoing surveillance imaging every 3 months to reevaluate his underlying disease burden, which has thus far been radiographically stable. Current management at the primary care level is focused on preserving quality of life, particularly maintaining mobility and functional independence.
Discussion
Although generally a benign entrapment neuropathy, MP bears well-established associations with multiple forms of must-not-miss pathology. Here, we present the case of a veteran in whom MP was the index presentation of a massive retroperitoneal liposarcoma, stressing the importance of a thorough history and physical examination in all patients presenting with MP. The case presented herein highlights many of the red-flag signs and symptoms that primary care physicians might encounter in patients with retroperitoneal pathology, including MP and MP-like syndromes (Figure 4).
In this case, the pretest probability of a spontaneous and uncomplicated MP was high given the patient’s sex, age, body habitus, and DM; however, there important atypia that emerged as the case evolved, including: (1) the progressive course; (2) proximal right lower extremity weakness; (3) asymmetric patellar reflexes; and (4) numerous clinical stigmata of intraabdominal mass effect. The patient exhibited abnormalities on abdominal examination that suggested the presence of an underlying intraabdominal mass, providing key diagnostic insight into this case. Given the slowly progressive nature of liposarcomas, we feel the abnormalities appreciated on abdominal examination were likely apparent during the initial presentation.18
There are numerous cognitive biases that may explain why an abdominal examination was not prioritized during the initial presentation. Namely, the patient’s numerous risk factors for spontaneous MP, as detailed above, may have contributed to framing bias that limited consideration of alternative diagnoses. In addition, the patient’s physical examination likely contributed to search satisfaction, whereby alternative diagnoses were not further entertained after discovery of findings consistent with spontaneous MP.19 Finally, it remains conceivable that an abdominal examination was not prioritized as it is often perceived as being distinct from, rather than an integral part of, the neurologic examination.20 Given that numerous neurologic disorders may present with abdominal pathology, we feel a thorough abdominal examination should be considered part of the full neurologic examination, especially in cases presenting with focal neurologic findings involving the lower extremities.21
Collectively, this case alludes to the importance of close clinical follow-up, as well as adequate anticipatory patient guidance in cases of suspected MP. In most patients, the clinical course of spontaneous MP is benign and favorable, with up to 85% of patients experiencing resolution within 4 to 6 months of the initial presentation.22 Common conservative measures include weight loss, garment optimization, and nonsteroidal anti-inflammatory drugs as needed for analgesia. In refractory cases, procedural interventions such as with neurolysis or resection of the lateral femoral cutaneous nerve, may be required after the ruling out of alternative diagnoses.23,24
Importantly, in even prolonged and resistant cases of MP, patient discomfort remains localized to the territory of the LFCN. Additional lower motor neuron signs, such as an expanding territory of sensory involvement, muscle weakness, or diminished reflexes, should prompt additional testing for alternative diagnoses. In addition, clinical findings concerning for intraabdominal mass effect, many of which were observed in this case, should lead to further evaluation and expeditious cross-sectional imaging. Although this patient’s early satiety, polyuria, bilateral lower extremity edema, weight gain, and lumbar plexopathy each may be explained by direct compression, invasion, or displacement, his report of progressive exertional dyspnea merits further discussion.
Exertional dyspnea is an uncommon complication of soft tissue sarcoma, reported almost exclusively in cases with cardiac, mediastinal, or other thoracic involvement.25-28 In this case, there was no evidence of thoracic involvement, either through direct extension or metastasis. Instead, the patient’s exertional dyspnea may have been attributable to increased intraabdominal pressure leading to compromised diaphragm excursion and reduced pulmonary reserve. In addition, the radiographic findings also raise the possibility of a potential contribution from preload failure due to IVC compression. Overall, dyspnea is a concerning feature that may suggest advanced disease.
Despite the value of a thorough history and physical examination in patients with MP, major clinical guidelines from neurologic, neurosurgical, and orthopedic organizations do not formally address MP evaluation and management. Further, proposed clinical practice algorithms are inconsistent in their recommendations regarding the identification of red-flag features and ruling out of alternative diagnoses.22,29,30 To supplement the abdominal examination, it would be reasonable to perform a pelvic compression test (PCT) in patients presenting with suspected MP. The PCT is a highly sensitive and specific provocative maneuver shown to enable reliable differentiation between MP and lumbar radiculopathy, and is performed by placing downward force on the anterior superior iliac spine of the affected extremity for 45 seconds with the patient in the lateral recumbent position.31 As this maneuver is intended to force relaxation of the inguinal ligament, thereby relieving pressure on the LFCN, improvement in the patient’s symptoms with the PCT is consistent with MP.
Conclusions
Meralgia paresthetica (MP) is a sensory mononeuropathy of the lateral femoral cutaneous nerve (LFCN), clinically characterized by numbness, pain, and paresthesias involving the anterolateral aspect of the thigh. Estimates of MP incidence are derived largely from observational studies and reported to be about 3.2 to 4.3 cases per 10,000 patient-years.1,2 Although typically arising during midlife and especially in the context of comorbid obesity, diabetes mellitus (DM), and excessive alcohol consumption, MP may occur at any age, and bears a slight predilection for males.2-4
MP may be divided etiologically into iatrogenic and spontaneous subtypes.5 Iatrogenic cases generally are attributable to nerve injury in the setting of direct or indirect trauma (such as with patient malpositioning) arising in the context of multiple forms of procedural or surgical intervention (Table). Spontaneous MP is primarily thought to occur as a result of LFCN compression at the level of the inguinal ligament, wherein internal or external pressures may promote LFCN entrapment and resultant functional disruption (Figure 1).6,7
External forces, such as tight garments, wallets, or even elements of modern body armor, have been reported to provoke MP.8-11 Alternatively, states of increased intraabdominal pressure, such as obesity, ascites, and pregnancy may predispose to LFCN compression.2,12,13 Less commonly, lumbar radiculopathy, pelvic masses, and several forms of retroperitoneal pathology may present with clinical symptomatology indistinguishable from MP.14-17 Importantly, many of these represent must-not-miss diagnoses, and may be suggested via a focused history and physical examination.
Here, we present a case of MP secondary to a massive retroperitoneal sarcoma, ultimately drawing renewed attention to the known association of MP and retroperitoneal pathology, and therein highlighting the utility of a dedicated review of systems to identify red-flag features in patients who present with MP and a thorough abdominal examination in all patients presenting with focal neurologic deficits involving the lower extremities.
Case Presentation
A male Vietnam War veteran aged 69 years presented to a primary care clinic at West Roxbury Veterans Affairs Medical Center (WRVAMC) in Massachusetts with progressive right lower extremity numbness. Three months prior to this visit, he was evaluated in an urgent care clinic at WRVAMC for 6 months of numbness and increasingly painful nocturnal paresthesias involving the same extremity. A targeted physical examination at that visit revealed an obese male wearing tight suspenders, as well as focally diminished sensation to light touch involving the anterolateral aspect of the thigh, extending from just below the right hip to above the knee. Sensation in the medial thigh was spared. Strength and reflexes were normal in the bilateral lower extremities. An abdominal examination was not performed. He received a diagnosis of MP and counseled regarding weight loss, glycemic control, garment optimization, and conservative analgesia with as-needed nonsteroidal anti-inflammatory drugs. He was instructed to follow-up closely with his primary care physician for further monitoring.
During the current visit, the patient reported 2 atraumatic falls the prior 2 months, attributed to escalating right leg weakness. The patient reported that ascending stairs had become difficult, and he was unable to cross his right leg over his left while in a seated position. The territory of numbness expanded to his front and inner thigh. Although previously he was able to hike 4 miles, he now was unable to walk more than half of a mile without developing shortness of breath. He reported frequent urination without hematuria and a recent weight gain of 8 pounds despite early satiety.
His medical history included hypertension, hypercholesterolemia, truncal obesity, noninsulin dependent DM, coronary artery disease, atrial flutter, transient ischemic attack, and benign positional paroxysmal vertigo. He was exposed to Agent Orange during his service in Vietnam. Family history was notable for breast cancer (mother), lung cancer (father), and an unspecified form of lymphoma (brother). He had smoked approximately 2 packs of cigarettes daily for 15 years but quit 38 years prior. He reported consuming on average 3 alcohol-containing drinks per week and no illicit drug use. He was adherent with all medications, including furosemide 40 mg daily, losartan 25 mg daily, metoprolol succinate 50 mg daily, atorvastatin 80 mg daily, metformin 500 mg twice daily, and rivaroxaban 20 mg daily with dinner.
His vital signs included a blood pressure of 123/58 mmHg, a pulse of 74 beats per minute, a respiratory rate of 16 breaths per minute, and an oxygen saturation of 94% on ambient air. His temperature was recorded at 96.7°F, and his weight was 234 pounds with a body mass index (BMI) of 34. He was well groomed and in no acute distress. His cardiopulmonary examination was normal. Carotid, radial, and bilateral dorsalis pedis pulsations were 2+ bilaterally, and no jugular venous distension was observed at 30°. The abdomen was protuberant. Nonshifting dullness to percussion and firmness to palpation was observed throughout right upper and lower quadrants, with hyperactive bowel sounds primarily localized to the left upper and lower quadrants.
Neurologic examination revealed symmetric facies with normal phonation and diction. He was spontaneously moving all extremities, and his gait was normal. Sensation to light touch was severely diminished throughout the anterolateral and medial thigh, extending to the level of the knee, and otherwise reduced in a stocking-type pattern over the bilateral feet and toes. His right hip flexion, adduction, as well as internal and external rotation were focally diminished to 4- out of 5. Right knee extension was 4+ out of 5. Strength was otherwise 5 out of 5. The patient exhibited asymmetric Patellar reflexes—absent on the right and 2+ on the left. Achilles reflexes were absent bilaterally. Straight-leg raise test was negative bilaterally and did not clearly exacerbate his right leg numbness or paresthesias. There were no notable fasciculations. There was 2+ bilateral lower extremity pitting edema appreciated to the level of the midshin (right greater than left), without palpable cords or new skin lesions.
Upon referral to the neurology service, the patient underwent electromyography, which revealed complex repetitive discharges in the right tibialis anterior and pattern of reduced recruitment upon activation of the right vastus medialis, collectively suggestive of an L3-4 plexopathy. The patient was admitted for expedited workup.
A complete blood count and metabolic panel that were taken in the emergency department were normal, save for a serum bicarbonate of 30 mEq/L. His hemoglobin A1c was 6.6%. Computed tomography (CT) of the abdomen and pelvis with IV contrast was obtained, and notable for a 30 cm fat-containing right-sided retroperitoneal mass with associated solid nodular components and calcification (Figure 2). No enhancement of the lesion was observed. There was significant associated mass effect, with superior displacement of the liver and right hemidiaphragm, as well as superomedial deflection of the right kidney, inferior vena cava, and other intraabdominal organs. Subsequent imaging with a CT of the chest, as well as magnetic resonance imaging of the brain, were without evidence of metastatic disease.
18Fluorodeoxyglucose-positron emission tomography (FDG-PET) was performed and demonstrated heterogeneous FDG avidity throughout the mass (SUVmax 5.9), as well as poor delineation of the boundary of the right psoas major, consistent with muscular invasion (Figure 3). The FDG-PET also revealed intense tracer uptake within the left prostate (SUVmax 26), concerning for a concomitant prostate malignancy.
To facilitate tissue diagnosis, the patient underwent a CT-guided biopsy of the retroperitoneal mass. Subsequent histopathologic analysis revealed a primarily well-differentiated spindle cell lesion with occasional adipocytic atypia, and a superimposed hypercellular element characterized by the presence of pleomorphic high-grade spindled cells. The neoplastic spindle cells were MDM2-positive by both immunohistochemistry and fluorescence in situ hybridization (FISH), and negative for pancytokeratin, smooth muscle myosin, and S100. The findings were collectively consistent with a dedifferentiated liposarcoma (DDLPS).
Given the focus of FDG avidity observed on the PET, the patient underwent a transrectal ultrasound-guided biopsy of the prostate, which yielded diagnosis of a concomitant high-risk (Gleason 4+4) prostate adenocarcinoma. A bone scan did not reveal evidence of osseous metastatic disease.
Outcome
The patient was treated with external beam radiotherapy (EBRT) delivered simultaneously to both the prostate and high-risk retroperitoneal margins of the DDLPS, as well as concurrent androgen deprivation therapy. Five months after completed radiotherapy, resection of the DDLPS was attempted. However, palliative tumor debulking was instead performed due to extensive locoregional invasion with involvement of the posterior peritoneum and ipsilateral quadratus, iliopsoas, and psoas muscles, as well as the adjacent lumbar nerve roots.
At present, the patient is undergoing surveillance imaging every 3 months to reevaluate his underlying disease burden, which has thus far been radiographically stable. Current management at the primary care level is focused on preserving quality of life, particularly maintaining mobility and functional independence.
Discussion
Although generally a benign entrapment neuropathy, MP bears well-established associations with multiple forms of must-not-miss pathology. Here, we present the case of a veteran in whom MP was the index presentation of a massive retroperitoneal liposarcoma, stressing the importance of a thorough history and physical examination in all patients presenting with MP. The case presented herein highlights many of the red-flag signs and symptoms that primary care physicians might encounter in patients with retroperitoneal pathology, including MP and MP-like syndromes (Figure 4).
In this case, the pretest probability of a spontaneous and uncomplicated MP was high given the patient’s sex, age, body habitus, and DM; however, there important atypia that emerged as the case evolved, including: (1) the progressive course; (2) proximal right lower extremity weakness; (3) asymmetric patellar reflexes; and (4) numerous clinical stigmata of intraabdominal mass effect. The patient exhibited abnormalities on abdominal examination that suggested the presence of an underlying intraabdominal mass, providing key diagnostic insight into this case. Given the slowly progressive nature of liposarcomas, we feel the abnormalities appreciated on abdominal examination were likely apparent during the initial presentation.18
There are numerous cognitive biases that may explain why an abdominal examination was not prioritized during the initial presentation. Namely, the patient’s numerous risk factors for spontaneous MP, as detailed above, may have contributed to framing bias that limited consideration of alternative diagnoses. In addition, the patient’s physical examination likely contributed to search satisfaction, whereby alternative diagnoses were not further entertained after discovery of findings consistent with spontaneous MP.19 Finally, it remains conceivable that an abdominal examination was not prioritized as it is often perceived as being distinct from, rather than an integral part of, the neurologic examination.20 Given that numerous neurologic disorders may present with abdominal pathology, we feel a thorough abdominal examination should be considered part of the full neurologic examination, especially in cases presenting with focal neurologic findings involving the lower extremities.21
Collectively, this case alludes to the importance of close clinical follow-up, as well as adequate anticipatory patient guidance in cases of suspected MP. In most patients, the clinical course of spontaneous MP is benign and favorable, with up to 85% of patients experiencing resolution within 4 to 6 months of the initial presentation.22 Common conservative measures include weight loss, garment optimization, and nonsteroidal anti-inflammatory drugs as needed for analgesia. In refractory cases, procedural interventions such as with neurolysis or resection of the lateral femoral cutaneous nerve, may be required after the ruling out of alternative diagnoses.23,24
Importantly, in even prolonged and resistant cases of MP, patient discomfort remains localized to the territory of the LFCN. Additional lower motor neuron signs, such as an expanding territory of sensory involvement, muscle weakness, or diminished reflexes, should prompt additional testing for alternative diagnoses. In addition, clinical findings concerning for intraabdominal mass effect, many of which were observed in this case, should lead to further evaluation and expeditious cross-sectional imaging. Although this patient’s early satiety, polyuria, bilateral lower extremity edema, weight gain, and lumbar plexopathy each may be explained by direct compression, invasion, or displacement, his report of progressive exertional dyspnea merits further discussion.
Exertional dyspnea is an uncommon complication of soft tissue sarcoma, reported almost exclusively in cases with cardiac, mediastinal, or other thoracic involvement.25-28 In this case, there was no evidence of thoracic involvement, either through direct extension or metastasis. Instead, the patient’s exertional dyspnea may have been attributable to increased intraabdominal pressure leading to compromised diaphragm excursion and reduced pulmonary reserve. In addition, the radiographic findings also raise the possibility of a potential contribution from preload failure due to IVC compression. Overall, dyspnea is a concerning feature that may suggest advanced disease.
Despite the value of a thorough history and physical examination in patients with MP, major clinical guidelines from neurologic, neurosurgical, and orthopedic organizations do not formally address MP evaluation and management. Further, proposed clinical practice algorithms are inconsistent in their recommendations regarding the identification of red-flag features and ruling out of alternative diagnoses.22,29,30 To supplement the abdominal examination, it would be reasonable to perform a pelvic compression test (PCT) in patients presenting with suspected MP. The PCT is a highly sensitive and specific provocative maneuver shown to enable reliable differentiation between MP and lumbar radiculopathy, and is performed by placing downward force on the anterior superior iliac spine of the affected extremity for 45 seconds with the patient in the lateral recumbent position.31 As this maneuver is intended to force relaxation of the inguinal ligament, thereby relieving pressure on the LFCN, improvement in the patient’s symptoms with the PCT is consistent with MP.
Conclusions
1. van Slobbe AM, Bohnen AM, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol. 2004;251(3):294-297. doi:10.1007/s00415-004-0310-x
2. Parisi TJ, Mandrekar J, Dyck PJ, Klein CJ. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology. 2011;77(16):1538-1542. doi:10.1212/WNL.0b013e318233b356
3. Ecker AD. Diagnosis of meralgia paresthetica. JAMA. 1985;253(7):976.
4. Massey EW, Pellock JM. Meralgia paraesthetica in a child. J Pediatr. 1978;93(2):325-326. doi:10.1016/s0022-3476(78)80566-6
5. Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8(8):669-677. doi:10.1111/j.1526-4637.2006.00227.x
6. Berini SE, Spinner RJ, Jentoft ME, et al. Chronic meralgia paresthetica and neurectomy: a clinical pathologic study. Neurology. 2014;82(17):1551-1555. doi:10.1212/WNL.0000000000000367
7. Payne RA, Harbaugh K, Specht CS, Rizk E. Correlation of histopathology and clinical symptoms in meralgia paresthetica. Cureus. 2017;9(10):e1789. Published 2017 Oct 20. doi:10.7759/cureus.1789
8. Boyce JR. Meralgia paresthetica and tight trousers. JAMA. 1984;251(12):1553.
9. Orton D. Meralgia paresthetica from a wallet. JAMA. 1984;252(24):3368.
10. Fargo MV, Konitzer LN. Meralgia paresthetica due to body armor wear in U.S. soldiers serving in Iraq: a case report and review of the literature. Mil Med. 2007;172(6):663-665. doi:10.7205/milmed.172.6.663
11. Korkmaz N, Ozçakar L. Meralgia paresthetica in a policeman: the belt or the gun. Plast Reconstr Surg. 2004;114(4):1012-1013. doi:10.1097/01.prs.0000138706.86633.01
12. Gooding MS, Evangelista V, Pereira L. Carpal Tunnel Syndrome and Meralgia Paresthetica in Pregnancy. Obstet Gynecol Surv. 2020;75(2):121-126. doi:10.1097/OGX.0000000000000745
13. Pauwels A, Amarenco P, Chazouillères O, Pigot F, Calmus Y, Lévy VG. Une complication rare et méconnue de l’ascite: la méralgie paresthésique [Unusual and unknown complication of ascites: meralgia paresthetica]. Gastroenterol Clin Biol. 1990;14(3):295.
14. Braddom RL. L2 rather than L1 radiculopathy mimics meralgia paresthetica. Muscle Nerve. 2010;42(5):842. doi:10.1002/mus.21826
15. Suber DA, Massey EW. Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol. 1979;53(2):257-258.
16. Flowers RS. Meralgia paresthetica. A clue to retroperitoneal malignant tumor. Am J Surg. 1968;116(1):89-92. doi:10.1016/0002-9610(68)90423-6
17. Yi TI, Yoon TH, Kim JS, Lee GE, Kim BR. Femoral neuropathy and meralgia paresthetica secondary to an iliacus hematoma. Ann Rehabil Med. 2012;36(2):273-277. doi:10.5535/arm.2012.36.2.273
18. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;36(2):151-159. doi:10.1200/JCO.2017.74.9598
19. O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48(3):225-232. doi:10.4997/JRCPE.2018.306
20. Bickley, LS. Bates’ Guide to Physical Examination and History Taking. 12th Edition. Wolters Kluwer Health/Lippincott Williams and Wilkins; 2016.
21. Bhavsar AS, Verma S, Lamba R, Lall CG, Koenigsknecht V, Rajesh A. Abdominal manifestations of neurologic disorders. Radiographics. 2013;33(1):135-153. doi:10.1148/rg.331125097
22. Dureja GP, Gulaya V, Jayalakshmi TS, Mandal P. Management of meralgia paresthetica: a multimodality regimen. Anesth Analg. 1995;80(5):1060-1061. doi:10.1097/00000539-199505000-00043
23. Patijn J, Mekhail N, Hayek S, Lataster A, van Kleef M, Van Zundert J. Meralgia paresthetica. Pain Pract. 2011;11(3):302-308. doi:10.1111/j.1533-2500.2011.00458.x24. Ivins GK. Meralgia paresthetica, the elusive diagnosis: clinical experience with 14 adult patients. Ann Surg. 2000;232(2):281-286. doi:10.1097/00000658-200008000-00019
25. Munin MA, Goerner MS, Raggio I, et al. A rare cause of dyspnea: undifferentiated pleomorphic sarcoma in the left atrium. Cardiol Res. 2017;8(5):241-245. doi:10.14740/cr590w
26. Nguyen A, Awad WI. Cardiac sarcoma arising from malignant transformation of a preexisting atrial myxoma. Ann Thorac Surg. 2016;101(4):1571-1573. doi:10.1016/j.athoracsur.2015.05.129
27. Jiang S, Li J, Zeng Q, Liang J. Pulmonary artery intimal sarcoma misdiagnosed as pulmonary embolism: a case report. Oncol Lett. 2017;13(4):2713-2716. doi:10.3892/ol.2017.5775
28. Cojocaru A, Oliveira PJ, Pellecchia C. A pleural presentation of a rare soft tissue sarcoma. Am J Resp Crit Care Med. 2012;185:A5201. doi:10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5201
29. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(5):336-344. doi:10.5435/00124635-200109000-00007
30. Cheatham SW, Kolber MJ, Salamh PA. Meralgia paresthetica: a review of the literature. Int J Sports Phys Ther. 2013;8(6):883-893.
31. Nouraei SA, Anand B, Spink G, O’Neill KS. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60(4):696-700. doi:10.1227/01.NEU.0000255392.69914.F7
32. Antunes PE, Antunes MJ. Meralgia paresthetica after aortic valve surgery. J Heart Valve Dis. 1997;6(6):589-590.
33. Reddy YM, Singh D, Chikkam V, et al. Postprocedural neuropathy after atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36(3):279-285. doi:10.1007/s10840-012-9724-z
34. Butler R, Webster MW. Meralgia paresthetica: an unusual complication of cardiac catheterization via the femoral artery. Catheter Cardiovasc Interv. 2002;56(1):69-71. doi:10.1002/ccd.10149
35. Jellish WS, Oftadeh M. Peripheral nerve injury in cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):495-511. doi:10.1053/j.jvca.2017.08.030
36. Parsonnet V, Karasakalides A, Gielchinsky I, Hochberg M, Hussain SM. Meralgia paresthetica after coronary bypass surgery. J Thorac Cardiovasc Surg. 1991;101(2):219-221.
37. Macgregor AM, Thoburn EK. Meralgia paresthetica following bariatric surgery. Obes Surg. 1999;9(4):364-368. doi:10.1381/096089299765552945
38. Grace DM. Meralgia paresthetica after gastroplasty for morbid obesity. Can J Surg. 1987;30(1):64-65.
39. Polidori L, Magarelli M, Tramutoli R. Meralgia paresthetica as a complication of laparoscopic appendectomy. Surg Endosc. 2003;17(5):832. doi:10.1007/s00464-002-4279-1
40. Yamout B, Tayyim A, Farhat W. Meralgia paresthetica as a complication of laparoscopic cholecystectomy. Clin Neurol Neurosurg. 1994;96(2):143-144. doi:10.1016/0303-8467(94)90048-5
41. Broin EO, Horner C, Mealy K, et al. Meralgia paraesthetica following laparoscopic inguinal hernia repair. an anatomical analysis. Surg Endosc. 1995;9(1):76-78. doi:10.1007/BF00187893
42. Eubanks S, Newman L 3rd, Goehring L, et al. Meralgia paresthetica: a complication of laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3(5):381-385.
43. Atamaz F, Hepgüler S, Karasu Z, Kilic M. Meralgia paresthetica after liver transplantation: a case report. Transplant Proc. 2005;37(10):4424-4425. doi:10.1016/j.transproceed.2005.11.047
44. Chung KH, Lee JY, Ko TK, et al. Meralgia paresthetica affecting parturient women who underwent cesarean section -a case report-. Korean J Anesthesiol. 2010;59 Suppl(Suppl):S86-S89. doi:10.4097/kjae.2010.59.S.S86
45. Hutchins FL Jr, Huggins J, Delaney ML. Laparoscopic myomectomy-an unusual cause of meralgia paresthetica. J Am Assoc Gynecol Laparosc. 1998;5(3):309-311. doi:10.1016/s1074-3804(98)80039-x
46. Jones CD, Guiot L, Portelli M, Bullen T, Skaife P. Two interesting cases of meralgia paraesthetica. Pain Physician. 2017;20(6):E987-E989.
47. Peters G, Larner AJ. Meralgia paresthetica following gynecologic and obstetric surgery. Int J Gynaecol Obstet. 2006;95(1):42-43. doi:10.1016/j.ijgo.2006.05.025
48. Kvarnström N, Järvholm S, Johannesson L, Dahm-Kähler P, Olausson M, Brännström M. Live donors of the initial observational study of uterus transplantation-psychological and medical follow-up until 1 year after surgery in the 9 cases. Transplantation. 2017;101(3):664-670. doi:10.1097/TP.0000000000001567
49. Goulding K, Beaulé PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468(9):2397-2404. doi:10.1007/s11999-010-1406-5
50. Yamamoto T, Nagira K, Kurosaka M. Meralgia paresthetica occurring 40 years after iliac bone graft harvesting: case report. Neurosurgery. 2001;49(6):1455-1457. doi:10.1097/00006123-200112000-00028
51. Roqueplan F, Porcher R, Hamzé B, et al. Long-term results of percutaneous resection and interstitial laser ablation of osteoid osteomas. Eur Radiol. 2010;20(1):209-217. doi:10.1007/s00330-009-1537-9
52. Gupta A, Muzumdar D, Ramani PS. Meralgia paraesthetica following lumbar spine surgery: a study in 110 consecutive surgically treated cases. Neurol India. 2004;52(1):64-66.
53. Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine (Phila Pa 1976). 2005;30(18):E547-E550. doi:10.1097/01.brs.0000178821.14102.9d
54. Tejwani SG, Scaduto AA, Bowen RE. Transient meralgia paresthetica after pediatric posterior spine fusion. J Pediatr Orthop. 2006;26(4):530-533. doi:10.1097/01.bpo.0000217721.95480.9e
55. Peker S, Ay B, Sun I, Ozgen S, Pamir M. Meralgia paraesthetica: complications of prone position during lumbar disc surgery. Internet J Anesthesiol. 2003;8(1):24-29.
1. van Slobbe AM, Bohnen AM, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol. 2004;251(3):294-297. doi:10.1007/s00415-004-0310-x
2. Parisi TJ, Mandrekar J, Dyck PJ, Klein CJ. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology. 2011;77(16):1538-1542. doi:10.1212/WNL.0b013e318233b356
3. Ecker AD. Diagnosis of meralgia paresthetica. JAMA. 1985;253(7):976.
4. Massey EW, Pellock JM. Meralgia paraesthetica in a child. J Pediatr. 1978;93(2):325-326. doi:10.1016/s0022-3476(78)80566-6
5. Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8(8):669-677. doi:10.1111/j.1526-4637.2006.00227.x
6. Berini SE, Spinner RJ, Jentoft ME, et al. Chronic meralgia paresthetica and neurectomy: a clinical pathologic study. Neurology. 2014;82(17):1551-1555. doi:10.1212/WNL.0000000000000367
7. Payne RA, Harbaugh K, Specht CS, Rizk E. Correlation of histopathology and clinical symptoms in meralgia paresthetica. Cureus. 2017;9(10):e1789. Published 2017 Oct 20. doi:10.7759/cureus.1789
8. Boyce JR. Meralgia paresthetica and tight trousers. JAMA. 1984;251(12):1553.
9. Orton D. Meralgia paresthetica from a wallet. JAMA. 1984;252(24):3368.
10. Fargo MV, Konitzer LN. Meralgia paresthetica due to body armor wear in U.S. soldiers serving in Iraq: a case report and review of the literature. Mil Med. 2007;172(6):663-665. doi:10.7205/milmed.172.6.663
11. Korkmaz N, Ozçakar L. Meralgia paresthetica in a policeman: the belt or the gun. Plast Reconstr Surg. 2004;114(4):1012-1013. doi:10.1097/01.prs.0000138706.86633.01
12. Gooding MS, Evangelista V, Pereira L. Carpal Tunnel Syndrome and Meralgia Paresthetica in Pregnancy. Obstet Gynecol Surv. 2020;75(2):121-126. doi:10.1097/OGX.0000000000000745
13. Pauwels A, Amarenco P, Chazouillères O, Pigot F, Calmus Y, Lévy VG. Une complication rare et méconnue de l’ascite: la méralgie paresthésique [Unusual and unknown complication of ascites: meralgia paresthetica]. Gastroenterol Clin Biol. 1990;14(3):295.
14. Braddom RL. L2 rather than L1 radiculopathy mimics meralgia paresthetica. Muscle Nerve. 2010;42(5):842. doi:10.1002/mus.21826
15. Suber DA, Massey EW. Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol. 1979;53(2):257-258.
16. Flowers RS. Meralgia paresthetica. A clue to retroperitoneal malignant tumor. Am J Surg. 1968;116(1):89-92. doi:10.1016/0002-9610(68)90423-6
17. Yi TI, Yoon TH, Kim JS, Lee GE, Kim BR. Femoral neuropathy and meralgia paresthetica secondary to an iliacus hematoma. Ann Rehabil Med. 2012;36(2):273-277. doi:10.5535/arm.2012.36.2.273
18. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;36(2):151-159. doi:10.1200/JCO.2017.74.9598
19. O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48(3):225-232. doi:10.4997/JRCPE.2018.306
20. Bickley, LS. Bates’ Guide to Physical Examination and History Taking. 12th Edition. Wolters Kluwer Health/Lippincott Williams and Wilkins; 2016.
21. Bhavsar AS, Verma S, Lamba R, Lall CG, Koenigsknecht V, Rajesh A. Abdominal manifestations of neurologic disorders. Radiographics. 2013;33(1):135-153. doi:10.1148/rg.331125097
22. Dureja GP, Gulaya V, Jayalakshmi TS, Mandal P. Management of meralgia paresthetica: a multimodality regimen. Anesth Analg. 1995;80(5):1060-1061. doi:10.1097/00000539-199505000-00043
23. Patijn J, Mekhail N, Hayek S, Lataster A, van Kleef M, Van Zundert J. Meralgia paresthetica. Pain Pract. 2011;11(3):302-308. doi:10.1111/j.1533-2500.2011.00458.x24. Ivins GK. Meralgia paresthetica, the elusive diagnosis: clinical experience with 14 adult patients. Ann Surg. 2000;232(2):281-286. doi:10.1097/00000658-200008000-00019
25. Munin MA, Goerner MS, Raggio I, et al. A rare cause of dyspnea: undifferentiated pleomorphic sarcoma in the left atrium. Cardiol Res. 2017;8(5):241-245. doi:10.14740/cr590w
26. Nguyen A, Awad WI. Cardiac sarcoma arising from malignant transformation of a preexisting atrial myxoma. Ann Thorac Surg. 2016;101(4):1571-1573. doi:10.1016/j.athoracsur.2015.05.129
27. Jiang S, Li J, Zeng Q, Liang J. Pulmonary artery intimal sarcoma misdiagnosed as pulmonary embolism: a case report. Oncol Lett. 2017;13(4):2713-2716. doi:10.3892/ol.2017.5775
28. Cojocaru A, Oliveira PJ, Pellecchia C. A pleural presentation of a rare soft tissue sarcoma. Am J Resp Crit Care Med. 2012;185:A5201. doi:10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5201
29. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(5):336-344. doi:10.5435/00124635-200109000-00007
30. Cheatham SW, Kolber MJ, Salamh PA. Meralgia paresthetica: a review of the literature. Int J Sports Phys Ther. 2013;8(6):883-893.
31. Nouraei SA, Anand B, Spink G, O’Neill KS. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60(4):696-700. doi:10.1227/01.NEU.0000255392.69914.F7
32. Antunes PE, Antunes MJ. Meralgia paresthetica after aortic valve surgery. J Heart Valve Dis. 1997;6(6):589-590.
33. Reddy YM, Singh D, Chikkam V, et al. Postprocedural neuropathy after atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36(3):279-285. doi:10.1007/s10840-012-9724-z
34. Butler R, Webster MW. Meralgia paresthetica: an unusual complication of cardiac catheterization via the femoral artery. Catheter Cardiovasc Interv. 2002;56(1):69-71. doi:10.1002/ccd.10149
35. Jellish WS, Oftadeh M. Peripheral nerve injury in cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):495-511. doi:10.1053/j.jvca.2017.08.030
36. Parsonnet V, Karasakalides A, Gielchinsky I, Hochberg M, Hussain SM. Meralgia paresthetica after coronary bypass surgery. J Thorac Cardiovasc Surg. 1991;101(2):219-221.
37. Macgregor AM, Thoburn EK. Meralgia paresthetica following bariatric surgery. Obes Surg. 1999;9(4):364-368. doi:10.1381/096089299765552945
38. Grace DM. Meralgia paresthetica after gastroplasty for morbid obesity. Can J Surg. 1987;30(1):64-65.
39. Polidori L, Magarelli M, Tramutoli R. Meralgia paresthetica as a complication of laparoscopic appendectomy. Surg Endosc. 2003;17(5):832. doi:10.1007/s00464-002-4279-1
40. Yamout B, Tayyim A, Farhat W. Meralgia paresthetica as a complication of laparoscopic cholecystectomy. Clin Neurol Neurosurg. 1994;96(2):143-144. doi:10.1016/0303-8467(94)90048-5
41. Broin EO, Horner C, Mealy K, et al. Meralgia paraesthetica following laparoscopic inguinal hernia repair. an anatomical analysis. Surg Endosc. 1995;9(1):76-78. doi:10.1007/BF00187893
42. Eubanks S, Newman L 3rd, Goehring L, et al. Meralgia paresthetica: a complication of laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3(5):381-385.
43. Atamaz F, Hepgüler S, Karasu Z, Kilic M. Meralgia paresthetica after liver transplantation: a case report. Transplant Proc. 2005;37(10):4424-4425. doi:10.1016/j.transproceed.2005.11.047
44. Chung KH, Lee JY, Ko TK, et al. Meralgia paresthetica affecting parturient women who underwent cesarean section -a case report-. Korean J Anesthesiol. 2010;59 Suppl(Suppl):S86-S89. doi:10.4097/kjae.2010.59.S.S86
45. Hutchins FL Jr, Huggins J, Delaney ML. Laparoscopic myomectomy-an unusual cause of meralgia paresthetica. J Am Assoc Gynecol Laparosc. 1998;5(3):309-311. doi:10.1016/s1074-3804(98)80039-x
46. Jones CD, Guiot L, Portelli M, Bullen T, Skaife P. Two interesting cases of meralgia paraesthetica. Pain Physician. 2017;20(6):E987-E989.
47. Peters G, Larner AJ. Meralgia paresthetica following gynecologic and obstetric surgery. Int J Gynaecol Obstet. 2006;95(1):42-43. doi:10.1016/j.ijgo.2006.05.025
48. Kvarnström N, Järvholm S, Johannesson L, Dahm-Kähler P, Olausson M, Brännström M. Live donors of the initial observational study of uterus transplantation-psychological and medical follow-up until 1 year after surgery in the 9 cases. Transplantation. 2017;101(3):664-670. doi:10.1097/TP.0000000000001567
49. Goulding K, Beaulé PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468(9):2397-2404. doi:10.1007/s11999-010-1406-5
50. Yamamoto T, Nagira K, Kurosaka M. Meralgia paresthetica occurring 40 years after iliac bone graft harvesting: case report. Neurosurgery. 2001;49(6):1455-1457. doi:10.1097/00006123-200112000-00028
51. Roqueplan F, Porcher R, Hamzé B, et al. Long-term results of percutaneous resection and interstitial laser ablation of osteoid osteomas. Eur Radiol. 2010;20(1):209-217. doi:10.1007/s00330-009-1537-9
52. Gupta A, Muzumdar D, Ramani PS. Meralgia paraesthetica following lumbar spine surgery: a study in 110 consecutive surgically treated cases. Neurol India. 2004;52(1):64-66.
53. Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine (Phila Pa 1976). 2005;30(18):E547-E550. doi:10.1097/01.brs.0000178821.14102.9d
54. Tejwani SG, Scaduto AA, Bowen RE. Transient meralgia paresthetica after pediatric posterior spine fusion. J Pediatr Orthop. 2006;26(4):530-533. doi:10.1097/01.bpo.0000217721.95480.9e
55. Peker S, Ay B, Sun I, Ozgen S, Pamir M. Meralgia paraesthetica: complications of prone position during lumbar disc surgery. Internet J Anesthesiol. 2003;8(1):24-29.