User login
FDA lifts partial hold on tazemetostat trials
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
Teva Announces FDA Approval of Ajovy (fremanezumab-vfrm)
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
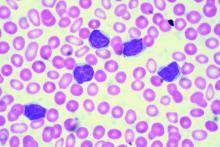
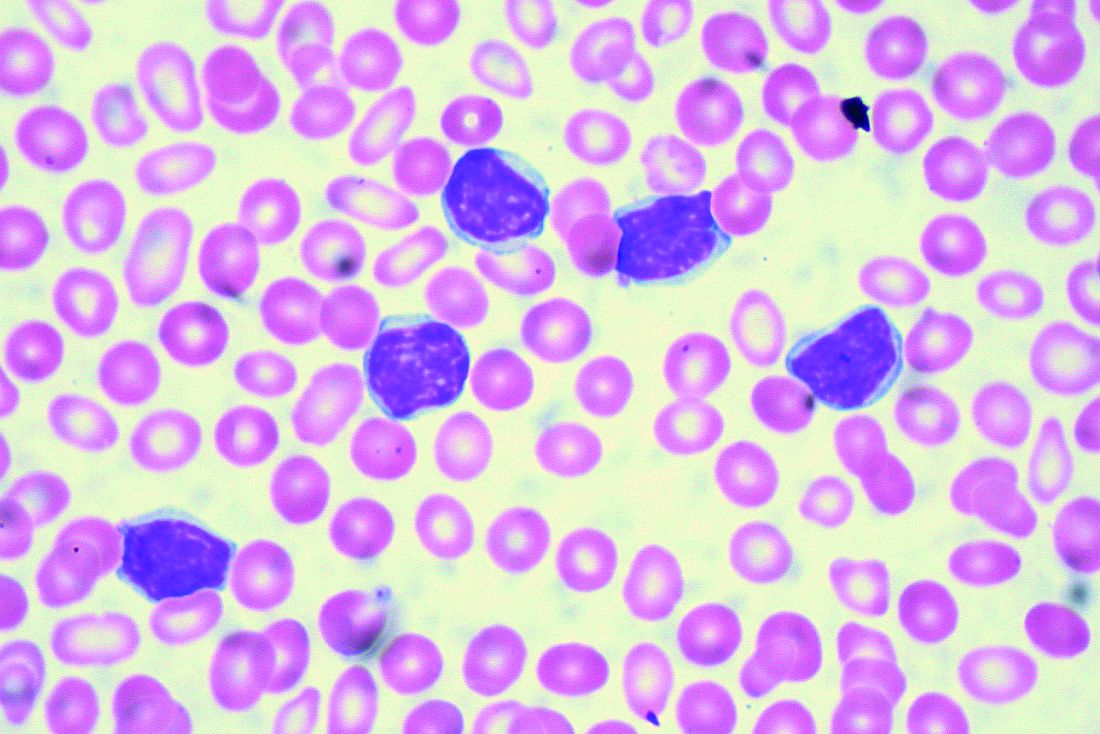
Real-world clues for optimal sequencing of CLL novel agents
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES CONGRESS
Outpatient lenalidomide/rituximab yields long-term MCL remission
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
After 5 years, the combination of lenalidomide and rituximab as first-line therapy for mantle cell lymphoma (MCL) continues to show durable responses with manageable toxicities, long-term results from a phase 2 clinical trial show.
After a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University, New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote. Their report was published in Blood.
In the multicenter, phase 2 single-arm study, 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase. Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P = .04).
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption. Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting, lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach,” the investigators wrote.
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
SOURCE: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
FROM BLOOD
Key clinical point:
Major finding: After 64-months of median follow-up, 21 of 33 patients with initial responses remained in remission.
Study details: Five-year follow-up of a phase 2 single arm trial of lenalidomide/rituximab induction and maintenance in 38 patients with mantle cell lymphoma.
Disclosures: The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation. Dr. Ruan has received research support and been a consultant for Celgene, and other coauthors reported research support and consultant relationships with the company.
Source: Ruan J et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-859769.
FDA approves new drug for CLL/SLL and follicular lymphoma
The Food and Drug Administration has approved duvelisib (Copiktra), a dual PI3K delta/gamma inhibitor, for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma.
who have received at least two prior therapies. Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint – in this case, overall response rate – that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem, the company marketing the drug. The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug – infections, diarrhea or colitis, cutaneous reactions, and pneumonitis. Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. The DUO trial included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2,000 mg).
Efficacy results are based on patients who had received at least two prior therapies, including 95 patients in the duvelisib arm and 101 in the ofatumumab arm. The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab.
The safety results include all patients treated with duvelisib or ofatumumab in this trial. In the duvelisib arm, 12% of patients had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab. Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection and diarrhea/colitis. The DYNAMO trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy. There were 83 patients with FL.
Patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
The Food and Drug Administration has approved duvelisib (Copiktra), a dual PI3K delta/gamma inhibitor, for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma.
who have received at least two prior therapies. Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint – in this case, overall response rate – that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem, the company marketing the drug. The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug – infections, diarrhea or colitis, cutaneous reactions, and pneumonitis. Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. The DUO trial included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2,000 mg).
Efficacy results are based on patients who had received at least two prior therapies, including 95 patients in the duvelisib arm and 101 in the ofatumumab arm. The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab.
The safety results include all patients treated with duvelisib or ofatumumab in this trial. In the duvelisib arm, 12% of patients had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab. Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection and diarrhea/colitis. The DYNAMO trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy. There were 83 patients with FL.
Patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
The Food and Drug Administration has approved duvelisib (Copiktra), a dual PI3K delta/gamma inhibitor, for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma.
who have received at least two prior therapies. Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint – in this case, overall response rate – that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem, the company marketing the drug. The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug – infections, diarrhea or colitis, cutaneous reactions, and pneumonitis. Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. The DUO trial included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2,000 mg).
Efficacy results are based on patients who had received at least two prior therapies, including 95 patients in the duvelisib arm and 101 in the ofatumumab arm. The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab.
The safety results include all patients treated with duvelisib or ofatumumab in this trial. In the duvelisib arm, 12% of patients had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab. Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection and diarrhea/colitis. The DYNAMO trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy. There were 83 patients with FL.
Patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
NICE looks likely to reject use of Kymriah for DLBCL
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).

Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).

Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
AYA cancer: Bridging the divide
and older adult patients, a trend that has been going on for decades. But clinicians and researchers are getting serious about an important question: Why?
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist, and director of pediatric bone marrow transplantation at Cleveland Clinic Children’s Hospital, Ohio, said in an interview.
He is referring to the cancers that affect adolescents and young adults (AYAs), who are broadly defined as patients aged 15-39 years.
“A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults; biology may be different in the adolescent and young adult patients, which may lead to different outcomes,” Dr. Hanna said.
In addition, the psychosocial needs in this age group differ vastly from those of other groups, he said.
“Many of these patients are in college or have just started their families, so we have to pay attention more to financial toxicities and fertility, for example,” he said.
Another factor that likely contributes to the disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs. That’s a point on which Dr. Hanna and many other experts agree.
A recent series of articles published in Blood addressed these and other issues, including whether AYAs with ALL or aggressive B-cell non-Hodgkin lymphomas (NHLs) should be treated as children or adults, treatment strategies for those with acute myeloid leukemias, management of Hodgkin lymphoma, and psychosocial challenges and health-related quality of life (QOL) of AYAs with hematologic malignancies.
“Hematological malignancies occurring in AYAs represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population,” Jorge Cortes, MD, an assistant editor for Blood, wrote in an introduction to the series.
“Unfortunately, not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs frequently centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults. Clinical trials specifically designed for AYAs are scanty,” noted Dr. Cortes, who directs the chronic myeloid leukemia (CML) and acute myeloid leukemia programs (AML) at the University of Texas MD Anderson Cancer Center, Houston.
Treatment approach and setting
In the Blood article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15-20 years is supported by numerous studies, and that in young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” as they have been shown to dramatically improve outcomes, with long-term survival rates nearing 70% (2018;132:351-61).
Patients in these age groups require specific programs that take into account factors such as care access and trial access, increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
Kristen O’Dwyer, MD, and her colleagues, in their article on AML treatment in AYAs, argue that based on “the distinguishing characteristics of AYAs with AML,” neither the pediatric nor adult approaches are ideally suited for them.
Rather, AYA-specific approaches merit consideration, they concluded (Blood 2018;132:362-68).
Similarly, Kieron Dunleavy, MD, and Thomas G. Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that a “remarkable divide” in the treatment of patients under age 18 years with lymphoma versus their young adult counterparts underscores the need for collaboration in developing consensus regarding treatment of AYAs (Blood 2018;132:369-75).
But recent findings from a study by Paul C. Nathan, MD, and his colleagues focuses more on where that treatment should take place (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy119).
The study provides new insights into the understanding of treatment differences for adolescents seen in pediatric vs. adult cancer facilities. And the findings suggest that the trade-off for improved outcomes among those treated in the pediatric setting – as emerging literature demonstrates – is higher resource use and cost, Helen M. Parsons, PhD, and her colleagues wrote in an accompanying editorial (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy123).
Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the cost of care was higher when treatment took place in a pediatric setting vs. an adult institution. This was driven in part by higher hospitalization rates and longer hospital stays, the investigators found.
“Additionally, adolescents treated in the pediatric setting tended to seek more [emergency department] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship,” Dr. Parsons and her colleagues wrote.
This was true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
The findings of higher inpatient days in the pediatric setting is not surprising given that induction therapies for pediatric ALL are generally more complex and intensive than therapies commonly used in adults with ALL, and given that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase ... more work on this topic is needed to more fully understand these patterns,” they wrote, adding that “the finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.”
Disease and developmental biology
As Dr. Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr. Dwyer and her colleagues said, explaining that a recent European study showed that in 5,564 patients with de novo AML, the frequency of favorable cytogenetics was low in infants, increased in children and young adults, and decreased again in middle age and older age (Cancer. 2016 Dec 15;122[24]:3821-30).
“Normal karyotype increases in prevalence from 13.7% in infants to approximately 25% in children, 44% in AYAs, and 50% in adults. Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr. Dwyer and her colleagues wrote, noting that it also is becoming more apparent that age influences the presence of AML-related molecular abnormalities.
The authors argue that recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr. Boissel and Dr. Baruchel also note that the “black hole” of understanding of ALL biology in AYAs that characterized the last 15 years has been “nearly brought to light and revealed a continuum between childhood and adult ALL.”
One example of this involves data from the NOPHO-ALL-2008 trial, showing that the proportion of patients with intrachromosomal amplification of the long arm of chromosome 21 (iAMP21), which is a rare event occurring in about 2% of children with ALL, is more frequent in older children and adolescents and is associated with higher relapse risk that is only partially diminished by intensified treatment.
In NOPHO-2008, iAMP21 occurred in 1.5% of patients aged 1-9 years, 5.8% of those aged 10-17 years, and 12% of those aged 17-45 years. The authors provided numerous other examples of such age-related differences in disease biology and concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
The “financial toxicity” mentioned by Dr. Hanna – the high cost of care, lost work time, and delays in reaching educational and career goals, for example – is a major factor that must be addressed in this population, but there are also many others.
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John M. Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, N.C., said in an interview.
These patients not only have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, but in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added.
“Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... These are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so,” he said.
In a 2014 study, he and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life. The latter finding was surprising, and highlights the “critical importance” of the social dimension in AYAs’ lives.
“Patient after patient will say ‘I found out who my real friends are,’ ” Dr. Salsman said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr. Salsman and his colleagues are using those findings to develop interventions that can maximize self care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves.
A randomized controlled pilot study incorporating social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions, for example, is underway.
Another intervention targets emotional well-being via web-based tools to increase positive affect. A proof-of-concept study showed that the approach is feasible and well received, and efforts are underway to plan a larger-scale randomized controlled trial, he said.
Dr. Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital.
PRISM was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis, and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr. Cortes noted a paucity of clinical trials specifically designed for this population.
“At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, adding that “enrollment of AYAs in clinical trials in cancer in general has been suboptimal at best.”
The dismal numbers with respect to enrollment of AYAs with cancer in clinical trials may be related in part to treatment setting, Dr. Salsman said.
Data suggest that the majority of AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers, where the bulk of research is being done, he explained.
The bottom line is that more research involving AYAs is needed, as is greater understanding of why enrollment is so much lower among AYA patients, Dr. Hanna said, noting that in 2017, The American Society of Clinical Oncology (ASCO) and Friends of Cancer Research (FOCR) released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.
Individuals aged 12 years and older should routinely be included in such trials as their drug metabolism is similar to that of adults, and inclusion of younger patients may also be appropriate if they are part of the population impacted by the disease, depending on specific disease biology, action of the drug, and available safety information, the organizations said.
Officials at the Food and Drug Administration are considering that possibility, Dr. Hanna said.
Attention to the disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups, has definitely increased in recent years, Dr. Salsman added, noting that in addition to ASCO and FOCR, several other organizations are working to address the problem.
About 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population; the Institute of Medicine held a forum on the care of AYAs with cancer; and the National Cancer Institute (NCI) held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology, he noted.
An article in Cancer provides a summary of the progress toward the priorities identified during the NCI meeting, which convened five working groups to address various topics, including clinical trial enrollment (Cancer. 2016 Apr 1;122[7]:988-99).
Dr. Hanna added that groups such as the Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now.
“One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically coordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr. Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.
What’s next?
Among the recommendations of the authors of the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams with involvement of multidisciplinary teams.
Many centers are already working on models for collaborative care, Dr. Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in “getting stakeholders on the same page, helping them have a shared vision, and working to maximize improvements in outcomes.”
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr. Hanna said. In the case of the community-powered advocacy organization Critical Mass, they succeeded in getting lawmakers to introduce a bill in the U.S. House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
and older adult patients, a trend that has been going on for decades. But clinicians and researchers are getting serious about an important question: Why?
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist, and director of pediatric bone marrow transplantation at Cleveland Clinic Children’s Hospital, Ohio, said in an interview.
He is referring to the cancers that affect adolescents and young adults (AYAs), who are broadly defined as patients aged 15-39 years.
“A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults; biology may be different in the adolescent and young adult patients, which may lead to different outcomes,” Dr. Hanna said.
In addition, the psychosocial needs in this age group differ vastly from those of other groups, he said.
“Many of these patients are in college or have just started their families, so we have to pay attention more to financial toxicities and fertility, for example,” he said.
Another factor that likely contributes to the disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs. That’s a point on which Dr. Hanna and many other experts agree.
A recent series of articles published in Blood addressed these and other issues, including whether AYAs with ALL or aggressive B-cell non-Hodgkin lymphomas (NHLs) should be treated as children or adults, treatment strategies for those with acute myeloid leukemias, management of Hodgkin lymphoma, and psychosocial challenges and health-related quality of life (QOL) of AYAs with hematologic malignancies.
“Hematological malignancies occurring in AYAs represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population,” Jorge Cortes, MD, an assistant editor for Blood, wrote in an introduction to the series.
“Unfortunately, not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs frequently centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults. Clinical trials specifically designed for AYAs are scanty,” noted Dr. Cortes, who directs the chronic myeloid leukemia (CML) and acute myeloid leukemia programs (AML) at the University of Texas MD Anderson Cancer Center, Houston.
Treatment approach and setting
In the Blood article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15-20 years is supported by numerous studies, and that in young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” as they have been shown to dramatically improve outcomes, with long-term survival rates nearing 70% (2018;132:351-61).
Patients in these age groups require specific programs that take into account factors such as care access and trial access, increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
Kristen O’Dwyer, MD, and her colleagues, in their article on AML treatment in AYAs, argue that based on “the distinguishing characteristics of AYAs with AML,” neither the pediatric nor adult approaches are ideally suited for them.
Rather, AYA-specific approaches merit consideration, they concluded (Blood 2018;132:362-68).
Similarly, Kieron Dunleavy, MD, and Thomas G. Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that a “remarkable divide” in the treatment of patients under age 18 years with lymphoma versus their young adult counterparts underscores the need for collaboration in developing consensus regarding treatment of AYAs (Blood 2018;132:369-75).
But recent findings from a study by Paul C. Nathan, MD, and his colleagues focuses more on where that treatment should take place (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy119).
The study provides new insights into the understanding of treatment differences for adolescents seen in pediatric vs. adult cancer facilities. And the findings suggest that the trade-off for improved outcomes among those treated in the pediatric setting – as emerging literature demonstrates – is higher resource use and cost, Helen M. Parsons, PhD, and her colleagues wrote in an accompanying editorial (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy123).
Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the cost of care was higher when treatment took place in a pediatric setting vs. an adult institution. This was driven in part by higher hospitalization rates and longer hospital stays, the investigators found.
“Additionally, adolescents treated in the pediatric setting tended to seek more [emergency department] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship,” Dr. Parsons and her colleagues wrote.
This was true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
The findings of higher inpatient days in the pediatric setting is not surprising given that induction therapies for pediatric ALL are generally more complex and intensive than therapies commonly used in adults with ALL, and given that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase ... more work on this topic is needed to more fully understand these patterns,” they wrote, adding that “the finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.”
Disease and developmental biology
As Dr. Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr. Dwyer and her colleagues said, explaining that a recent European study showed that in 5,564 patients with de novo AML, the frequency of favorable cytogenetics was low in infants, increased in children and young adults, and decreased again in middle age and older age (Cancer. 2016 Dec 15;122[24]:3821-30).
“Normal karyotype increases in prevalence from 13.7% in infants to approximately 25% in children, 44% in AYAs, and 50% in adults. Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr. Dwyer and her colleagues wrote, noting that it also is becoming more apparent that age influences the presence of AML-related molecular abnormalities.
The authors argue that recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr. Boissel and Dr. Baruchel also note that the “black hole” of understanding of ALL biology in AYAs that characterized the last 15 years has been “nearly brought to light and revealed a continuum between childhood and adult ALL.”
One example of this involves data from the NOPHO-ALL-2008 trial, showing that the proportion of patients with intrachromosomal amplification of the long arm of chromosome 21 (iAMP21), which is a rare event occurring in about 2% of children with ALL, is more frequent in older children and adolescents and is associated with higher relapse risk that is only partially diminished by intensified treatment.
In NOPHO-2008, iAMP21 occurred in 1.5% of patients aged 1-9 years, 5.8% of those aged 10-17 years, and 12% of those aged 17-45 years. The authors provided numerous other examples of such age-related differences in disease biology and concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
The “financial toxicity” mentioned by Dr. Hanna – the high cost of care, lost work time, and delays in reaching educational and career goals, for example – is a major factor that must be addressed in this population, but there are also many others.
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John M. Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, N.C., said in an interview.
These patients not only have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, but in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added.
“Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... These are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so,” he said.
In a 2014 study, he and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life. The latter finding was surprising, and highlights the “critical importance” of the social dimension in AYAs’ lives.
“Patient after patient will say ‘I found out who my real friends are,’ ” Dr. Salsman said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr. Salsman and his colleagues are using those findings to develop interventions that can maximize self care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves.
A randomized controlled pilot study incorporating social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions, for example, is underway.
Another intervention targets emotional well-being via web-based tools to increase positive affect. A proof-of-concept study showed that the approach is feasible and well received, and efforts are underway to plan a larger-scale randomized controlled trial, he said.
Dr. Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital.
PRISM was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis, and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr. Cortes noted a paucity of clinical trials specifically designed for this population.
“At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, adding that “enrollment of AYAs in clinical trials in cancer in general has been suboptimal at best.”
The dismal numbers with respect to enrollment of AYAs with cancer in clinical trials may be related in part to treatment setting, Dr. Salsman said.
Data suggest that the majority of AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers, where the bulk of research is being done, he explained.
The bottom line is that more research involving AYAs is needed, as is greater understanding of why enrollment is so much lower among AYA patients, Dr. Hanna said, noting that in 2017, The American Society of Clinical Oncology (ASCO) and Friends of Cancer Research (FOCR) released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.
Individuals aged 12 years and older should routinely be included in such trials as their drug metabolism is similar to that of adults, and inclusion of younger patients may also be appropriate if they are part of the population impacted by the disease, depending on specific disease biology, action of the drug, and available safety information, the organizations said.
Officials at the Food and Drug Administration are considering that possibility, Dr. Hanna said.
Attention to the disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups, has definitely increased in recent years, Dr. Salsman added, noting that in addition to ASCO and FOCR, several other organizations are working to address the problem.
About 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population; the Institute of Medicine held a forum on the care of AYAs with cancer; and the National Cancer Institute (NCI) held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology, he noted.
An article in Cancer provides a summary of the progress toward the priorities identified during the NCI meeting, which convened five working groups to address various topics, including clinical trial enrollment (Cancer. 2016 Apr 1;122[7]:988-99).
Dr. Hanna added that groups such as the Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now.
“One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically coordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr. Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.
What’s next?
Among the recommendations of the authors of the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams with involvement of multidisciplinary teams.
Many centers are already working on models for collaborative care, Dr. Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in “getting stakeholders on the same page, helping them have a shared vision, and working to maximize improvements in outcomes.”
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr. Hanna said. In the case of the community-powered advocacy organization Critical Mass, they succeeded in getting lawmakers to introduce a bill in the U.S. House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
and older adult patients, a trend that has been going on for decades. But clinicians and researchers are getting serious about an important question: Why?
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist, and director of pediatric bone marrow transplantation at Cleveland Clinic Children’s Hospital, Ohio, said in an interview.
He is referring to the cancers that affect adolescents and young adults (AYAs), who are broadly defined as patients aged 15-39 years.
“A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults; biology may be different in the adolescent and young adult patients, which may lead to different outcomes,” Dr. Hanna said.
In addition, the psychosocial needs in this age group differ vastly from those of other groups, he said.
“Many of these patients are in college or have just started their families, so we have to pay attention more to financial toxicities and fertility, for example,” he said.
Another factor that likely contributes to the disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs. That’s a point on which Dr. Hanna and many other experts agree.
A recent series of articles published in Blood addressed these and other issues, including whether AYAs with ALL or aggressive B-cell non-Hodgkin lymphomas (NHLs) should be treated as children or adults, treatment strategies for those with acute myeloid leukemias, management of Hodgkin lymphoma, and psychosocial challenges and health-related quality of life (QOL) of AYAs with hematologic malignancies.
“Hematological malignancies occurring in AYAs represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population,” Jorge Cortes, MD, an assistant editor for Blood, wrote in an introduction to the series.
“Unfortunately, not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs frequently centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults. Clinical trials specifically designed for AYAs are scanty,” noted Dr. Cortes, who directs the chronic myeloid leukemia (CML) and acute myeloid leukemia programs (AML) at the University of Texas MD Anderson Cancer Center, Houston.
Treatment approach and setting
In the Blood article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15-20 years is supported by numerous studies, and that in young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” as they have been shown to dramatically improve outcomes, with long-term survival rates nearing 70% (2018;132:351-61).
Patients in these age groups require specific programs that take into account factors such as care access and trial access, increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
Kristen O’Dwyer, MD, and her colleagues, in their article on AML treatment in AYAs, argue that based on “the distinguishing characteristics of AYAs with AML,” neither the pediatric nor adult approaches are ideally suited for them.
Rather, AYA-specific approaches merit consideration, they concluded (Blood 2018;132:362-68).
Similarly, Kieron Dunleavy, MD, and Thomas G. Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that a “remarkable divide” in the treatment of patients under age 18 years with lymphoma versus their young adult counterparts underscores the need for collaboration in developing consensus regarding treatment of AYAs (Blood 2018;132:369-75).
But recent findings from a study by Paul C. Nathan, MD, and his colleagues focuses more on where that treatment should take place (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy119).
The study provides new insights into the understanding of treatment differences for adolescents seen in pediatric vs. adult cancer facilities. And the findings suggest that the trade-off for improved outcomes among those treated in the pediatric setting – as emerging literature demonstrates – is higher resource use and cost, Helen M. Parsons, PhD, and her colleagues wrote in an accompanying editorial (J Natl Cancer Inst. 2018 Jul 19. doi: 10.1093/jnci/djy123).
Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the cost of care was higher when treatment took place in a pediatric setting vs. an adult institution. This was driven in part by higher hospitalization rates and longer hospital stays, the investigators found.
“Additionally, adolescents treated in the pediatric setting tended to seek more [emergency department] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship,” Dr. Parsons and her colleagues wrote.
This was true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
The findings of higher inpatient days in the pediatric setting is not surprising given that induction therapies for pediatric ALL are generally more complex and intensive than therapies commonly used in adults with ALL, and given that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase ... more work on this topic is needed to more fully understand these patterns,” they wrote, adding that “the finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.”
Disease and developmental biology
As Dr. Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr. Dwyer and her colleagues said, explaining that a recent European study showed that in 5,564 patients with de novo AML, the frequency of favorable cytogenetics was low in infants, increased in children and young adults, and decreased again in middle age and older age (Cancer. 2016 Dec 15;122[24]:3821-30).
“Normal karyotype increases in prevalence from 13.7% in infants to approximately 25% in children, 44% in AYAs, and 50% in adults. Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr. Dwyer and her colleagues wrote, noting that it also is becoming more apparent that age influences the presence of AML-related molecular abnormalities.
The authors argue that recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr. Boissel and Dr. Baruchel also note that the “black hole” of understanding of ALL biology in AYAs that characterized the last 15 years has been “nearly brought to light and revealed a continuum between childhood and adult ALL.”
One example of this involves data from the NOPHO-ALL-2008 trial, showing that the proportion of patients with intrachromosomal amplification of the long arm of chromosome 21 (iAMP21), which is a rare event occurring in about 2% of children with ALL, is more frequent in older children and adolescents and is associated with higher relapse risk that is only partially diminished by intensified treatment.
In NOPHO-2008, iAMP21 occurred in 1.5% of patients aged 1-9 years, 5.8% of those aged 10-17 years, and 12% of those aged 17-45 years. The authors provided numerous other examples of such age-related differences in disease biology and concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
The “financial toxicity” mentioned by Dr. Hanna – the high cost of care, lost work time, and delays in reaching educational and career goals, for example – is a major factor that must be addressed in this population, but there are also many others.
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John M. Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, N.C., said in an interview.
These patients not only have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, but in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added.
“Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... These are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so,” he said.
In a 2014 study, he and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life. The latter finding was surprising, and highlights the “critical importance” of the social dimension in AYAs’ lives.
“Patient after patient will say ‘I found out who my real friends are,’ ” Dr. Salsman said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr. Salsman and his colleagues are using those findings to develop interventions that can maximize self care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves.
A randomized controlled pilot study incorporating social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions, for example, is underway.
Another intervention targets emotional well-being via web-based tools to increase positive affect. A proof-of-concept study showed that the approach is feasible and well received, and efforts are underway to plan a larger-scale randomized controlled trial, he said.
Dr. Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital.
PRISM was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis, and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr. Cortes noted a paucity of clinical trials specifically designed for this population.
“At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, adding that “enrollment of AYAs in clinical trials in cancer in general has been suboptimal at best.”
The dismal numbers with respect to enrollment of AYAs with cancer in clinical trials may be related in part to treatment setting, Dr. Salsman said.
Data suggest that the majority of AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers, where the bulk of research is being done, he explained.
The bottom line is that more research involving AYAs is needed, as is greater understanding of why enrollment is so much lower among AYA patients, Dr. Hanna said, noting that in 2017, The American Society of Clinical Oncology (ASCO) and Friends of Cancer Research (FOCR) released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.
Individuals aged 12 years and older should routinely be included in such trials as their drug metabolism is similar to that of adults, and inclusion of younger patients may also be appropriate if they are part of the population impacted by the disease, depending on specific disease biology, action of the drug, and available safety information, the organizations said.
Officials at the Food and Drug Administration are considering that possibility, Dr. Hanna said.
Attention to the disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups, has definitely increased in recent years, Dr. Salsman added, noting that in addition to ASCO and FOCR, several other organizations are working to address the problem.
About 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population; the Institute of Medicine held a forum on the care of AYAs with cancer; and the National Cancer Institute (NCI) held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology, he noted.
An article in Cancer provides a summary of the progress toward the priorities identified during the NCI meeting, which convened five working groups to address various topics, including clinical trial enrollment (Cancer. 2016 Apr 1;122[7]:988-99).
Dr. Hanna added that groups such as the Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now.
“One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically coordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr. Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.
What’s next?
Among the recommendations of the authors of the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams with involvement of multidisciplinary teams.
Many centers are already working on models for collaborative care, Dr. Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in “getting stakeholders on the same page, helping them have a shared vision, and working to maximize improvements in outcomes.”
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr. Hanna said. In the case of the community-powered advocacy organization Critical Mass, they succeeded in getting lawmakers to introduce a bill in the U.S. House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
CK and TP53 status should be assessed together, team says
Researchers say they have found evidence to suggest that patients with mantle cell lymphoma (MCL) should be evaluated for TP53 mutation and complex karyotype (CK) simultaneously before treatment.
The team’s study showed that TP53 mutation and CK occurred independently, but patients with both characteristics had poor prognosis.
All patients with TP53 mutation and CK died within 1.2 years of diagnosis, whereas about 94% of patients with neither characteristic were still alive at 2 years.
The researchers also found that, by combining TP53 mutation and CK, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment.
Vít Procházka, MD, PhD, of Palacký University in Olomouc, Czech Republic, and his colleagues reported these findings in Clinical Lymphoma, Myeloma & Leukemia.
The study included 74 consecutive adults newly diagnosed with MCL from 2000 through 2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or CK, was under observation without therapy.
Altogether, 48 patients (64.9%) had biological material available to perform analyses for TP53 mutation and CK. Of those, 4 patients were found to have both TP53 mutation and CK, 12 had one of the two markers, and 32 had neither.
While all patients with both markers died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker (P<0.001).
Progression-free survival analyses showed similar results. The 2-year progression-free survival rate was 41.7% for patients with one marker and 78% for patients with neither marker (P<0.001).
Multivariate analysis showed that both TP53 mutation and CK were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index.
While larger studies are needed to confirm these results, the researchers suggested that novel treatment approaches might be warranted for patients in the highest risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and CK might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” the researchers said.
This study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
Researchers say they have found evidence to suggest that patients with mantle cell lymphoma (MCL) should be evaluated for TP53 mutation and complex karyotype (CK) simultaneously before treatment.
The team’s study showed that TP53 mutation and CK occurred independently, but patients with both characteristics had poor prognosis.
All patients with TP53 mutation and CK died within 1.2 years of diagnosis, whereas about 94% of patients with neither characteristic were still alive at 2 years.
The researchers also found that, by combining TP53 mutation and CK, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment.
Vít Procházka, MD, PhD, of Palacký University in Olomouc, Czech Republic, and his colleagues reported these findings in Clinical Lymphoma, Myeloma & Leukemia.
The study included 74 consecutive adults newly diagnosed with MCL from 2000 through 2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or CK, was under observation without therapy.
Altogether, 48 patients (64.9%) had biological material available to perform analyses for TP53 mutation and CK. Of those, 4 patients were found to have both TP53 mutation and CK, 12 had one of the two markers, and 32 had neither.
While all patients with both markers died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker (P<0.001).
Progression-free survival analyses showed similar results. The 2-year progression-free survival rate was 41.7% for patients with one marker and 78% for patients with neither marker (P<0.001).
Multivariate analysis showed that both TP53 mutation and CK were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index.
While larger studies are needed to confirm these results, the researchers suggested that novel treatment approaches might be warranted for patients in the highest risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and CK might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” the researchers said.
This study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
Researchers say they have found evidence to suggest that patients with mantle cell lymphoma (MCL) should be evaluated for TP53 mutation and complex karyotype (CK) simultaneously before treatment.
The team’s study showed that TP53 mutation and CK occurred independently, but patients with both characteristics had poor prognosis.
All patients with TP53 mutation and CK died within 1.2 years of diagnosis, whereas about 94% of patients with neither characteristic were still alive at 2 years.
The researchers also found that, by combining TP53 mutation and CK, patients could be stratified into three prognostic groups that had distinct outcomes, regardless of treatment.
Vít Procházka, MD, PhD, of Palacký University in Olomouc, Czech Republic, and his colleagues reported these findings in Clinical Lymphoma, Myeloma & Leukemia.
The study included 74 consecutive adults newly diagnosed with MCL from 2000 through 2014. Seventy-three patients were treated with a rituximab-containing regimen. One patient, who did not have TP53 mutation or CK, was under observation without therapy.
Altogether, 48 patients (64.9%) had biological material available to perform analyses for TP53 mutation and CK. Of those, 4 patients were found to have both TP53 mutation and CK, 12 had one of the two markers, and 32 had neither.
While all patients with both markers died within 1.2 years, 2-year overall survival was 50.0% for those with one marker and 93.8% for those with neither marker (P<0.001).
Progression-free survival analyses showed similar results. The 2-year progression-free survival rate was 41.7% for patients with one marker and 78% for patients with neither marker (P<0.001).
Multivariate analysis showed that both TP53 mutation and CK were predictors of inferior progression-free survival and overall survival, independent of age and scores on the MCL International Prognostic Index.
While larger studies are needed to confirm these results, the researchers suggested that novel treatment approaches might be warranted for patients in the highest risk subgroup.
“The patients harboring the negative prognostic markers [TP53 mutation] and CK might be indicated for a novel induction treatment strategy probably in combination with maintenance therapy different from rituximab,” the researchers said.
This study was supported by grants from the Czech Ministry of Health and Palacký University. The researchers reported having no conflicts of interest.
NICE rejects DLBCL indication for CAR T-cell therapy
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
Risks of watchful waiting in follicular lymphoma
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.
A subset of follicular lymphoma (FL) patients managed with watchful waiting are vulnerable to organ dysfunction and transformation, according to research published in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study, about 24% of FL patients managed with watchful waiting developed significant organ dysfunction or transformation at first progression over 8.2 years of follow-up.
Organ dysfunction and transformation were associated with significantly worse overall survival (OS) that could not be predicted based on baseline characteristics.
Gwynivere A. Davies, MD, of the University of Calgary in Alberta, Canada, and her colleagues conducted this study using data from the Alberta Lymphoma Database. The team gathered data on patients with grade 1-3a FL who were diagnosed between 1994 and 2011.
The investigators identified 238 patients who were initially managed with watchful waiting. The patients had a median age of 54.1 years (range, 24.7-69.9) at diagnosis, and 83.2% were advanced stage.
The 10-year OS rate for these patients was 81.2%. At a median follow-up of 98.5 months, 71% (n=169) of patients had progressed and required therapy.
At the time of progression, 24.4% of patients (n=58) had organ dysfunction and/or transformation. The median time to organ dysfunction/transformation was 29.9 months.
These adverse outcomes were significantly associated with inferior OS. The 10-year OS rate was 65.4% for patients with transformation at progression and 83.2% for those without transformation (P=0.0017).
The 10-year OS rate was 71.5% for those with organ dysfunction at progression and 82.7% for those without organ dysfunction (P=0.028).
Comparison to treated patients
The investigators also looked at a comparison group of 236 FL patients managed with immediate rituximab-based chemotherapy (R-chemo), most of whom were scheduled to receive (72.9%) rituximab maintenance. Their median age was 52.1 (range, 27.3-65.4), and most (82.6%) had advanced stage disease.
At a median follow-up of 100.2 months, the median progression-free survival (PFS) was not reached. The 10-year OS rate was 84%.
The 10-year PFS rate after first R-chemo was 57.1% for patients who received immediate R-chemo (n=236) and 50.5% for patients who were initially managed with watchful waiting and proceeded to R-chemo (n=133; P=0.506). This was not affected by rituximab maintenance.
The investigators noted that OS measured from diagnosis was not affected by initial watchful waiting.
However, in a landmark analysis, OS was inferior when measured from R-chemo at first progression for watchful waiting recipients compared to patients who received immediate R-chemo. The 10-year OS rates were 74.4% and 84.0%, respectively (P=0.02).
The risk of transformation at first progression was significantly different between the groups. At 10 years, the rate of transformation was 25.5% in the watchful waiting group and 6.3% in the immediate R-chemo group (P<0.0001).
The investigators said these findings, taken together, suggest changes may be warranted for FL patients managed with watchful waiting.
“Consideration should be given to implementing standardized follow-up imaging, with early initiation of rituximab-based therapy if there is evidence of progression in an attempt to prevent these potentially clinically impactful events [i.e., organ dysfunction and transformation],” Dr. Davies and her coauthors wrote.
Dr. Davies reported no financial disclosures. Her coauthors reported disclosures related to Janssen, Gilead Sciences, Lundbeck, Roche, AbbVie, Amgen, Seattle Genetics, Bristol-Myers Squibb, Servier Laboratories, and Merck.