User login
Staging PET/CT better defines extent of mantle cell lymphoma
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
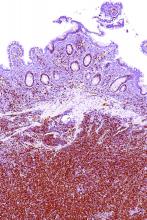
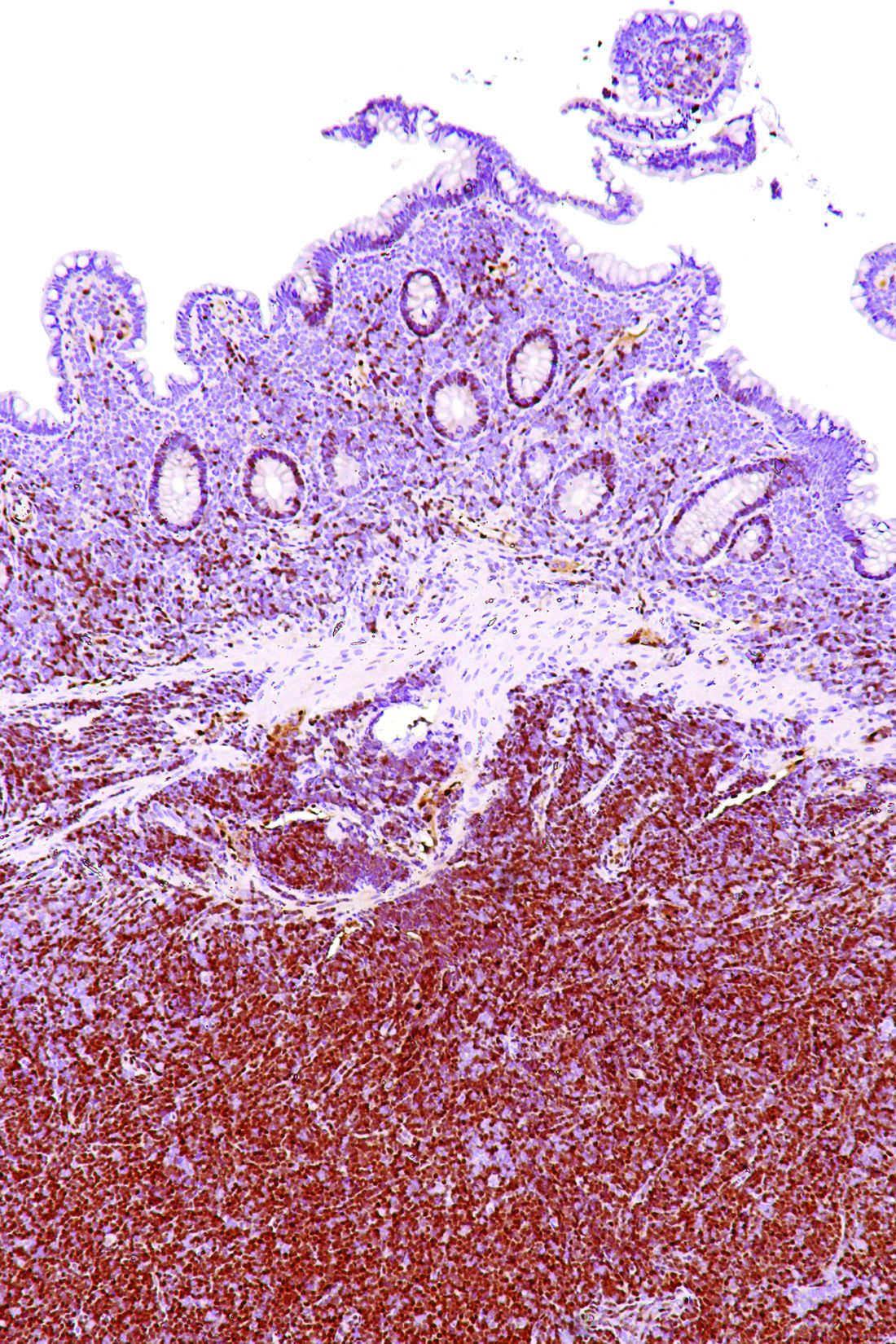
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
FROM CLINICAL LYMPHOMA, MYELOMA, & LEUKEMIA
Could home care replace inpatient HSCT?
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Study confirms prognostic impact of MYC partner gene in DLBCL
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Can a novel steroidal anti-inflammatory drug benefit patients with Duchenne muscular dystrophy?
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
FROM NEUROLOGY
Interview with Andrew Solomon, MD, on diagnosing multiple sclerosis
Andrew Solomon, MD, is a neurologist and Associate Professor in the Department of Neurological Sciences and Division Chief of Multiple Sclerosis at The University of Vermont. We sat down to talk with Dr. Solomon about his experience with multiple sclerosis (MS) misdiagnosis and what can be done to improve MS diagnosis going forward.
How prevalent is the misdiagnosis of MS and what are the effects that it has on patients?
The first thing to clarify is what we mean by MS misdiagnosis. In this case, we’re talking about patients who are incorrectly assigned a diagnosis of MS. MS is hard to diagnose. Sometimes we take too long to diagnose people who have MS, sometimes we incorrectly diagnose MS in people who don’t have it.
We don’t have very good data in terms of how frequent misdiagnosis is, but we have some. The earliest data we have is from the 1980s. In 1988 there was a study published involving approximately 500 patients who had been diagnosed with MS in life and subsequently died between the 1960s and the 1980s and had a postmortem exam. 6% of those people who had a diagnosis of MS during their lifetime didn’t actually have MS.1
In 2012, we did a survey of 122 MS specialists.2 We asked them if they had encountered patients incorrectly assigned a diagnosis of MS in the past year, and 95% of them had seen such a patient. This was not the most scientific study because it was just a survey and subject to recall bias. Still, many of these MS providers recalled having seen three to ten such patients in the last year where they strongly felt that a pre-existing MS diagnosis made by another provider was incorrect.
Another study was recently published by Dr. Kaisey.3 She looked at referrals to two academic MS centers on the West Coast, and specifically new patient referrals over 12 months to those two MS centers. She found that out of 241 patients referred to the two MS centers, almost one in five who was referred with a pre-existing diagnosis of MS who came to these MS centers was subsequently found not have MS. This included 17% of patients at Cedars Sinai and 19% at UCLA. That’s an alarmingly high number of patients. And that’s the best data we have right now.
We don’t know how representative that number is of other MS centers and clinical practice nationally, but the bottom line is that misdiagnosis of MS is, unfortunately, fairly common and there’s a lot of risks to patients associated with misdiagnosis, as well as costs to our health care system.
Can you elaborate on the risks to patients?
We published a study where we looked at records from 110 patients who had been incorrectly assigned a diagnosis of MS. Twenty-three MS specialists from 4 different MS centers participated in this study that was published in Neurology in 2016.4
70% of these patients had been receiving disease modifying therapy for MS, which certainly has risks and side effects associated with it. 24% received disease modifying therapy with a known risk of PML, which is a frequently fatal brain infection associated with these therapies. Approximately 30% of these patients who did not have MS were on disease modifying therapy for in 3 to 9 years and 30% were on disease modifying therapy for 10 years or more.
Dr. Kaisey’s study also supports our findings. She found approximately 110 patient-years of exposure to unnecessary disease modifying therapy in the misdiagnosed patients in her study.3 Patients suffer side effects in addition to unnecessary risk related to these therapies.
It’s also important to emphasize that many of these patients also received inadequate treatment for their correct diagnoses.
How did the 2017 revision to the McDonald criteria address the challenge of MS diagnosis?
The problem of MS misdiagnosis is prominently discussed in the 2017 McDonald criteria.5 Unfortunately, one of the likely causes of misdiagnosis is that many clinicians may not read the full manuscript of the McDonald criteria itself. They instead rely on summary cards or reproductions—condensed versions of how to diagnose MS—which often don’t really provide the full context that would allow physicians to think critically and avoid a misdiagnosis.
MS is still a clinical diagnosis, which means we’re reliant on physician decision-making to confirm a diagnosis of MS. There are multiple steps to it.
First, we must determine if somebody has a syndrome typical for MS and they have objective evidence of a CNS lesion. After that the McDonald criteria can be applied to determine if they meet dissemination in time, dissemination in space, and have no other explanation for this syndrome before making a diagnosis of MS. Each one of those steps is reliant on thoughtful clinical decision-making and may be prone to potential error.
Knowing the ins and outs and the details of each of those steps is important. Reading the diagnostic criteria is probably the first step in becoming skilled at MS diagnosis. It’s important to know the criteria were not developed as a screening tool. The neurologist is essentially the screening tool. The diagnostic criteria can’t be used until a MS-typical syndrome with objective evidence of a CNS lesion is confirmed.
In what way was the previous criteria flawed?
I wouldn’t say any of the MS diagnostic criteria were flawed; they have evolved along with data in our field that has helped us make diagnosis of MS earlier in many patients. When using the criteria, it’s important to understand the types of patients in the cohorts used to validate our diagnostic criteria. They were primarily younger than 50, and usually Caucasian. They had only syndromes typical for MS with objective evidence of CNS damage corroborating these syndromes. Using the criteria more broadly in patients who do not fit this profile can reduce its specificity and lead to misdiagnosis.
For determination of MRI dissemination in time and dissemination space, there are also some misconceptions that frequently lead to misdiagnosis. Knowing which areas are required for dissemination in space in crucial. For example, the optic nerve currently is not an area that can be used to fulfill dissemination in space, which is a mistake people frequently make. Knowing that the terms “juxtacortical” and “periventricular” means touching the ventricle and touching the cortex is very important. This is a mistake that’s often made as well, and many disorders present with MRI lesions near but not touching the cortex or ventricle. Knowing each element of our diagnostic criteria and what those terms specifically mean is important. In the 2017 McDonald criteria there’s an excellent glossary that helps clinicians understand these terms and how to use them appropriately.5
What more needs to be done to prevent MS misdiagnosis?
First, we need to figure out how to better educate clinicians on how to use our diagnostic criteria appropriately.
We recently completed a study that suggests that residents in training, and even MS specialists, have trouble using the diagnostic criteria. This study was presented at the American Academy of Neurology Annual meeting but has not been published yet. Education on how to use the diagnostic criteria, and in which patients to use the criteria (and in which patients the criteria do not apply) is important, particularly when new revisions to the diagnostic criteria are published.
We published a paper recently that provided guidance on how to avoid misdiagnosis using the 2017 McDonald criteria, and how to approach patients where the diagnostic criteria didn’t apply.6 Sometimes additional clinical, laboratory, or MRI evaluation and monitoring is required in such patients to either confirm a diagnosis of MS, or determine that a patient does not have MS.
We also desperately need biomarkers that may help us diagnose MS more accurately in patients who have neurological symptoms and an abnormal MRI and are seeing a neurologist for the first time. There’s research going on now to find relevant biomarkers in the form of blood tests, as well as MRI assessments. In particular, ongoing research focused on the MRI finding we have termed the “central vein sign” suggests this approach may be helpful as a MRI-specific biomarker for MS.7,8 We need multicenter studies evaluating this and other biomarkers in patients who come to our clinics for a new evaluation for MS, to confirm that they are accurate. We need more researchers working on how to improve diagnosis of MS.
References:
1. Engell T. A clinico-pathoanatomical study of multiple sclerosis diagnosis. Acta Neurol Scand. 1988;78(1):39-44.
2. Solomon AJ, Klein EP, Bourdette D. "Undiagnosing" multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78(24):1986-1991.
3. Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord. 2019;30:51-56.
4. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
5. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173..
6. Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019;92(1):26-33.
7. Sati P, Oh J, Constable RT, et al; NAIMS Cooperative. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-722.
8. Sinnecker T, Clarke MA, Meier D, et al. Evaluation of the central vein sign as a diagnostic imaging biomarker in multiple sclerosis [published online ahead of print August 19, 2019]. JAMA Neurology. 2019: doi: 10.1001/jamaneurol.2019.2478.
Andrew Solomon, MD, is a neurologist and Associate Professor in the Department of Neurological Sciences and Division Chief of Multiple Sclerosis at The University of Vermont. We sat down to talk with Dr. Solomon about his experience with multiple sclerosis (MS) misdiagnosis and what can be done to improve MS diagnosis going forward.
How prevalent is the misdiagnosis of MS and what are the effects that it has on patients?
The first thing to clarify is what we mean by MS misdiagnosis. In this case, we’re talking about patients who are incorrectly assigned a diagnosis of MS. MS is hard to diagnose. Sometimes we take too long to diagnose people who have MS, sometimes we incorrectly diagnose MS in people who don’t have it.
We don’t have very good data in terms of how frequent misdiagnosis is, but we have some. The earliest data we have is from the 1980s. In 1988 there was a study published involving approximately 500 patients who had been diagnosed with MS in life and subsequently died between the 1960s and the 1980s and had a postmortem exam. 6% of those people who had a diagnosis of MS during their lifetime didn’t actually have MS.1
In 2012, we did a survey of 122 MS specialists.2 We asked them if they had encountered patients incorrectly assigned a diagnosis of MS in the past year, and 95% of them had seen such a patient. This was not the most scientific study because it was just a survey and subject to recall bias. Still, many of these MS providers recalled having seen three to ten such patients in the last year where they strongly felt that a pre-existing MS diagnosis made by another provider was incorrect.
Another study was recently published by Dr. Kaisey.3 She looked at referrals to two academic MS centers on the West Coast, and specifically new patient referrals over 12 months to those two MS centers. She found that out of 241 patients referred to the two MS centers, almost one in five who was referred with a pre-existing diagnosis of MS who came to these MS centers was subsequently found not have MS. This included 17% of patients at Cedars Sinai and 19% at UCLA. That’s an alarmingly high number of patients. And that’s the best data we have right now.
We don’t know how representative that number is of other MS centers and clinical practice nationally, but the bottom line is that misdiagnosis of MS is, unfortunately, fairly common and there’s a lot of risks to patients associated with misdiagnosis, as well as costs to our health care system.
Can you elaborate on the risks to patients?
We published a study where we looked at records from 110 patients who had been incorrectly assigned a diagnosis of MS. Twenty-three MS specialists from 4 different MS centers participated in this study that was published in Neurology in 2016.4
70% of these patients had been receiving disease modifying therapy for MS, which certainly has risks and side effects associated with it. 24% received disease modifying therapy with a known risk of PML, which is a frequently fatal brain infection associated with these therapies. Approximately 30% of these patients who did not have MS were on disease modifying therapy for in 3 to 9 years and 30% were on disease modifying therapy for 10 years or more.
Dr. Kaisey’s study also supports our findings. She found approximately 110 patient-years of exposure to unnecessary disease modifying therapy in the misdiagnosed patients in her study.3 Patients suffer side effects in addition to unnecessary risk related to these therapies.
It’s also important to emphasize that many of these patients also received inadequate treatment for their correct diagnoses.
How did the 2017 revision to the McDonald criteria address the challenge of MS diagnosis?
The problem of MS misdiagnosis is prominently discussed in the 2017 McDonald criteria.5 Unfortunately, one of the likely causes of misdiagnosis is that many clinicians may not read the full manuscript of the McDonald criteria itself. They instead rely on summary cards or reproductions—condensed versions of how to diagnose MS—which often don’t really provide the full context that would allow physicians to think critically and avoid a misdiagnosis.
MS is still a clinical diagnosis, which means we’re reliant on physician decision-making to confirm a diagnosis of MS. There are multiple steps to it.
First, we must determine if somebody has a syndrome typical for MS and they have objective evidence of a CNS lesion. After that the McDonald criteria can be applied to determine if they meet dissemination in time, dissemination in space, and have no other explanation for this syndrome before making a diagnosis of MS. Each one of those steps is reliant on thoughtful clinical decision-making and may be prone to potential error.
Knowing the ins and outs and the details of each of those steps is important. Reading the diagnostic criteria is probably the first step in becoming skilled at MS diagnosis. It’s important to know the criteria were not developed as a screening tool. The neurologist is essentially the screening tool. The diagnostic criteria can’t be used until a MS-typical syndrome with objective evidence of a CNS lesion is confirmed.
In what way was the previous criteria flawed?
I wouldn’t say any of the MS diagnostic criteria were flawed; they have evolved along with data in our field that has helped us make diagnosis of MS earlier in many patients. When using the criteria, it’s important to understand the types of patients in the cohorts used to validate our diagnostic criteria. They were primarily younger than 50, and usually Caucasian. They had only syndromes typical for MS with objective evidence of CNS damage corroborating these syndromes. Using the criteria more broadly in patients who do not fit this profile can reduce its specificity and lead to misdiagnosis.
For determination of MRI dissemination in time and dissemination space, there are also some misconceptions that frequently lead to misdiagnosis. Knowing which areas are required for dissemination in space in crucial. For example, the optic nerve currently is not an area that can be used to fulfill dissemination in space, which is a mistake people frequently make. Knowing that the terms “juxtacortical” and “periventricular” means touching the ventricle and touching the cortex is very important. This is a mistake that’s often made as well, and many disorders present with MRI lesions near but not touching the cortex or ventricle. Knowing each element of our diagnostic criteria and what those terms specifically mean is important. In the 2017 McDonald criteria there’s an excellent glossary that helps clinicians understand these terms and how to use them appropriately.5
What more needs to be done to prevent MS misdiagnosis?
First, we need to figure out how to better educate clinicians on how to use our diagnostic criteria appropriately.
We recently completed a study that suggests that residents in training, and even MS specialists, have trouble using the diagnostic criteria. This study was presented at the American Academy of Neurology Annual meeting but has not been published yet. Education on how to use the diagnostic criteria, and in which patients to use the criteria (and in which patients the criteria do not apply) is important, particularly when new revisions to the diagnostic criteria are published.
We published a paper recently that provided guidance on how to avoid misdiagnosis using the 2017 McDonald criteria, and how to approach patients where the diagnostic criteria didn’t apply.6 Sometimes additional clinical, laboratory, or MRI evaluation and monitoring is required in such patients to either confirm a diagnosis of MS, or determine that a patient does not have MS.
We also desperately need biomarkers that may help us diagnose MS more accurately in patients who have neurological symptoms and an abnormal MRI and are seeing a neurologist for the first time. There’s research going on now to find relevant biomarkers in the form of blood tests, as well as MRI assessments. In particular, ongoing research focused on the MRI finding we have termed the “central vein sign” suggests this approach may be helpful as a MRI-specific biomarker for MS.7,8 We need multicenter studies evaluating this and other biomarkers in patients who come to our clinics for a new evaluation for MS, to confirm that they are accurate. We need more researchers working on how to improve diagnosis of MS.
References:
1. Engell T. A clinico-pathoanatomical study of multiple sclerosis diagnosis. Acta Neurol Scand. 1988;78(1):39-44.
2. Solomon AJ, Klein EP, Bourdette D. "Undiagnosing" multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78(24):1986-1991.
3. Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord. 2019;30:51-56.
4. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
5. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173..
6. Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019;92(1):26-33.
7. Sati P, Oh J, Constable RT, et al; NAIMS Cooperative. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-722.
8. Sinnecker T, Clarke MA, Meier D, et al. Evaluation of the central vein sign as a diagnostic imaging biomarker in multiple sclerosis [published online ahead of print August 19, 2019]. JAMA Neurology. 2019: doi: 10.1001/jamaneurol.2019.2478.
Andrew Solomon, MD, is a neurologist and Associate Professor in the Department of Neurological Sciences and Division Chief of Multiple Sclerosis at The University of Vermont. We sat down to talk with Dr. Solomon about his experience with multiple sclerosis (MS) misdiagnosis and what can be done to improve MS diagnosis going forward.
How prevalent is the misdiagnosis of MS and what are the effects that it has on patients?
The first thing to clarify is what we mean by MS misdiagnosis. In this case, we’re talking about patients who are incorrectly assigned a diagnosis of MS. MS is hard to diagnose. Sometimes we take too long to diagnose people who have MS, sometimes we incorrectly diagnose MS in people who don’t have it.
We don’t have very good data in terms of how frequent misdiagnosis is, but we have some. The earliest data we have is from the 1980s. In 1988 there was a study published involving approximately 500 patients who had been diagnosed with MS in life and subsequently died between the 1960s and the 1980s and had a postmortem exam. 6% of those people who had a diagnosis of MS during their lifetime didn’t actually have MS.1
In 2012, we did a survey of 122 MS specialists.2 We asked them if they had encountered patients incorrectly assigned a diagnosis of MS in the past year, and 95% of them had seen such a patient. This was not the most scientific study because it was just a survey and subject to recall bias. Still, many of these MS providers recalled having seen three to ten such patients in the last year where they strongly felt that a pre-existing MS diagnosis made by another provider was incorrect.
Another study was recently published by Dr. Kaisey.3 She looked at referrals to two academic MS centers on the West Coast, and specifically new patient referrals over 12 months to those two MS centers. She found that out of 241 patients referred to the two MS centers, almost one in five who was referred with a pre-existing diagnosis of MS who came to these MS centers was subsequently found not have MS. This included 17% of patients at Cedars Sinai and 19% at UCLA. That’s an alarmingly high number of patients. And that’s the best data we have right now.
We don’t know how representative that number is of other MS centers and clinical practice nationally, but the bottom line is that misdiagnosis of MS is, unfortunately, fairly common and there’s a lot of risks to patients associated with misdiagnosis, as well as costs to our health care system.
Can you elaborate on the risks to patients?
We published a study where we looked at records from 110 patients who had been incorrectly assigned a diagnosis of MS. Twenty-three MS specialists from 4 different MS centers participated in this study that was published in Neurology in 2016.4
70% of these patients had been receiving disease modifying therapy for MS, which certainly has risks and side effects associated with it. 24% received disease modifying therapy with a known risk of PML, which is a frequently fatal brain infection associated with these therapies. Approximately 30% of these patients who did not have MS were on disease modifying therapy for in 3 to 9 years and 30% were on disease modifying therapy for 10 years or more.
Dr. Kaisey’s study also supports our findings. She found approximately 110 patient-years of exposure to unnecessary disease modifying therapy in the misdiagnosed patients in her study.3 Patients suffer side effects in addition to unnecessary risk related to these therapies.
It’s also important to emphasize that many of these patients also received inadequate treatment for their correct diagnoses.
How did the 2017 revision to the McDonald criteria address the challenge of MS diagnosis?
The problem of MS misdiagnosis is prominently discussed in the 2017 McDonald criteria.5 Unfortunately, one of the likely causes of misdiagnosis is that many clinicians may not read the full manuscript of the McDonald criteria itself. They instead rely on summary cards or reproductions—condensed versions of how to diagnose MS—which often don’t really provide the full context that would allow physicians to think critically and avoid a misdiagnosis.
MS is still a clinical diagnosis, which means we’re reliant on physician decision-making to confirm a diagnosis of MS. There are multiple steps to it.
First, we must determine if somebody has a syndrome typical for MS and they have objective evidence of a CNS lesion. After that the McDonald criteria can be applied to determine if they meet dissemination in time, dissemination in space, and have no other explanation for this syndrome before making a diagnosis of MS. Each one of those steps is reliant on thoughtful clinical decision-making and may be prone to potential error.
Knowing the ins and outs and the details of each of those steps is important. Reading the diagnostic criteria is probably the first step in becoming skilled at MS diagnosis. It’s important to know the criteria were not developed as a screening tool. The neurologist is essentially the screening tool. The diagnostic criteria can’t be used until a MS-typical syndrome with objective evidence of a CNS lesion is confirmed.
In what way was the previous criteria flawed?
I wouldn’t say any of the MS diagnostic criteria were flawed; they have evolved along with data in our field that has helped us make diagnosis of MS earlier in many patients. When using the criteria, it’s important to understand the types of patients in the cohorts used to validate our diagnostic criteria. They were primarily younger than 50, and usually Caucasian. They had only syndromes typical for MS with objective evidence of CNS damage corroborating these syndromes. Using the criteria more broadly in patients who do not fit this profile can reduce its specificity and lead to misdiagnosis.
For determination of MRI dissemination in time and dissemination space, there are also some misconceptions that frequently lead to misdiagnosis. Knowing which areas are required for dissemination in space in crucial. For example, the optic nerve currently is not an area that can be used to fulfill dissemination in space, which is a mistake people frequently make. Knowing that the terms “juxtacortical” and “periventricular” means touching the ventricle and touching the cortex is very important. This is a mistake that’s often made as well, and many disorders present with MRI lesions near but not touching the cortex or ventricle. Knowing each element of our diagnostic criteria and what those terms specifically mean is important. In the 2017 McDonald criteria there’s an excellent glossary that helps clinicians understand these terms and how to use them appropriately.5
What more needs to be done to prevent MS misdiagnosis?
First, we need to figure out how to better educate clinicians on how to use our diagnostic criteria appropriately.
We recently completed a study that suggests that residents in training, and even MS specialists, have trouble using the diagnostic criteria. This study was presented at the American Academy of Neurology Annual meeting but has not been published yet. Education on how to use the diagnostic criteria, and in which patients to use the criteria (and in which patients the criteria do not apply) is important, particularly when new revisions to the diagnostic criteria are published.
We published a paper recently that provided guidance on how to avoid misdiagnosis using the 2017 McDonald criteria, and how to approach patients where the diagnostic criteria didn’t apply.6 Sometimes additional clinical, laboratory, or MRI evaluation and monitoring is required in such patients to either confirm a diagnosis of MS, or determine that a patient does not have MS.
We also desperately need biomarkers that may help us diagnose MS more accurately in patients who have neurological symptoms and an abnormal MRI and are seeing a neurologist for the first time. There’s research going on now to find relevant biomarkers in the form of blood tests, as well as MRI assessments. In particular, ongoing research focused on the MRI finding we have termed the “central vein sign” suggests this approach may be helpful as a MRI-specific biomarker for MS.7,8 We need multicenter studies evaluating this and other biomarkers in patients who come to our clinics for a new evaluation for MS, to confirm that they are accurate. We need more researchers working on how to improve diagnosis of MS.
References:
1. Engell T. A clinico-pathoanatomical study of multiple sclerosis diagnosis. Acta Neurol Scand. 1988;78(1):39-44.
2. Solomon AJ, Klein EP, Bourdette D. "Undiagnosing" multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78(24):1986-1991.
3. Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord. 2019;30:51-56.
4. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
5. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173..
6. Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019;92(1):26-33.
7. Sati P, Oh J, Constable RT, et al; NAIMS Cooperative. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-722.
8. Sinnecker T, Clarke MA, Meier D, et al. Evaluation of the central vein sign as a diagnostic imaging biomarker in multiple sclerosis [published online ahead of print August 19, 2019]. JAMA Neurology. 2019: doi: 10.1001/jamaneurol.2019.2478.
Rituximab, bendamustine look better than chemo alone in MCL
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Migraines linked to higher risk of dementia
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
FROM THE INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY
CK doesn’t seem to affect OS in CLL patients taking idelalisib
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
FROM LEUKEMIA
Is serum serotonin level associated with risk of seizure-related breathing dysfunction?
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
FROM NEUROLOGY
Key clinical point: Significant increases in serum serotonin after a seizure are associated with lower risk of seizure-related breathing dysfunction.
Major finding: In patients without ictal central apnea, mean interictal serotonin level was 109.1 ng/mL, and postictal levels were 139.8 ng/mL.
Study details: A prospective cohort study of 49 patients with intractable epilepsy.
Disclosures: The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
Source: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
Open Clinical Trials for Patients With Lung Cancers (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors or cosponsors nearly 1,000 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of August 1, 201 8 ; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Lung-MAP (multiple trials)
Lung-MAP (SWOG S1400) is a multidrug, multi-substudy, biomarker-driven squamous cell lung cancer clinical trial that uses state-of-the-art genomic profiling to match patients to substudies testing investigational treatments that may target the genomic alterations, or mutations, found to be driving the growth of their cancer.
ID: NCT02154490, NCT02595944, NCT02766335, NCT02785913, NCT02785939, NCT02926638, NCT02965378, NCT03373760, NCT03377556
Sponsor: Southwest Oncology Group
Locations: VA Connecticut Healthcare System-West Haven Campus; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Ann Arbor VAMC, Michigan; Kansas City VAMC, Missouri; VA New Jersey Health Care System, East Orange; Michael E. DeBakey VAMC Houston, Texas
ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (multiple trials)
A group of randomized clinical trials for patients with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery.
ID: NCT02193282, NCT02194738, NCT02201992, NCT02595944
Sponsor: National Cancer Institute
Locations: Little Rock VAMC, Arkansas; VA Connecticut Healthcare System West Haven Campus; Atlanta VAMC, Decatur, Georgia; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; Minneapolis VAMC, Minnesota; Saint Louis VAMC, Missouri; Veterans Affairs New York Harbor Healthcare System-Brooklyn Campus; Dayton VAMC, Ohio; William S. Middleton VAMC, Madison, Wisconsin
Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggests that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of two incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these two treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life-expectancy, and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: Edward Hines Jr. VA Hospital, Hines, Illinois; Richard L. Roudebush VA Medical Center, Indianapolis, Indiana; Minneapolis VA Health Care System, Minnesota; Durham VAMC, North Carolina; Michael E. DeBakey VAMC, Houston, Texas; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Naloxegol in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer
This randomized pilot clinical trial studies the side effects and best dose of naloxegol and to see how well it works in treating patients with stage IIIB-IV non-small cell lung cancer. Naloxegol may relieve some of the side effects of opioid pain medication and fight off future growth in the cancer.
ID: NCT03087708
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Minneapolis VAMC, Minnesota; Kansas City VAMC, Missouri; VA Western New York Health Care System-Buffalo; Salisbury VAMC, North Carolina
Palliative Care Interventions for Outpatients Newly Diagnosed With Lung Cancer: Phase II (PCI2)
The focus of the study is to test a nurse-led telephone-based palliative care intervention on improving the delivery of care for patients with newly diagnosed lung cancer. The study is a three site randomized control trial to determine the efficacy of the intervention on improving patients’ quality of life, symptom burden, and satisfaction of care. Additionally, the study will test an innovative care delivery model to improve patients’ access to palliative care. The investigators will also determine the effect of the intervention on patient activation to discuss treatment preferences with their clinician and on clinician knowledge of patients’ goals of care.
ID: NCT03007953
Sponsor: VA Office of Research and Development
Locations: Birmingham VAMC, Alabama; VA Portland Health Care System, Oregon; VA Puget Sound Health Care System Seattle Division, Washington
Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide
Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as etoposide, carboplatin and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known which radiation therapy regimen is more effective when given together with chemotherapy in treating patients with limited-stage small cell lung cancer. This randomized phase III trial is comparing different chest radiation therapy regimens to see how well they work in treating patients with limited-stage small cell lung cancer.
ID: NCT00632853
Sponsor: Alliance for Clinical Trials in Oncology
Locations: Baltimore VAMC, Maryland; Kansas City VAMC, Missouri; VA Western New York Health Care System, Buffalo, New York; Dayton VAMC, Ohio; Zablocki VAMC, Milwaukee, Wisconsin
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer
Wedge resection or segmentectomy may be less invasive types of surgery than lobectomy for non-small cell lung cancer and may have fewer side effects and improve recovery. It is not yet known whether wedge resection or segmentectomy are more effective than lobectomy in treating stage IA non-small cell lung cancer.
ID: NCT00499330
Sponsor: Alliance for Clinical Trials in Oncology
Locations: VA Loma Linda Healthcare System, California; VA Long Beach Medical Center, California; Richard L. Roudebush VAMC, Indianapolis, Indiana; Portland VAMC, Oregon
Lung Cancer Screening Decisions (VA-LCSDecTool)
Veterans have a high risk of developing lung in comparison to general populations due to their older age and smoking history. Recent evidence indicates that lung cancer screening with low dose CT scan reduces lung cancer mortality among older heavy smokers. However, the rates of false positive findings are high, requiring further testing and evaluation. Preliminary studies report that while some Veterans are enthusiastic about screening, others are highly reluctant. Patient preferences should be considered as part of an informed decision making process for this emerging paradigm of lung cancer control. Effective methods for preference assessment among Veterans have not yet been developed, evaluated, and integrated into clinical practice. The specific aims of this study are to 1) elicit patient and provider stakeholder input to inform the development of a lung cancer screening decision tool, 2) develop a web based Lung Cancer Screening Decision Tool (LCSDecTool) that incorporates patient and provider input, and 3) evaluate the impact of the LCSDecTool compared to usual care on the decision process, clinical outcomes, and quality of life.
ID
Sponsor: VA Office of Research and Development
Locations: VA Connecticut Healthcare System West Haven Campus; Corporal Michael J. Crescenz VAMC Philadelphia, Pennsylvania
Molecular Predictors of Cancer in Patients at High Risk of Lung Cancer
Using samples of blood, urine, sputum, and lung tissue from patients at high risk of cancer for laboratory studies may help doctors learn more about changes that may occur in DNA and identify biomarkers related to cancer.
ID: NCT00898313
Sponsor: Vanderbilt-Ingram Cancer Center
Location: VAMC Nashville, Tennessee
Improving Supportive Care for Patients With Thoracic Malignancies
The purpose of this study is to use a proactive approach to improve symptom management of patients with thoracic malignancies. In this pilot study, the investigators propose to evaluate the feasibility of using outbound, proactive telephone symptom assessment strategies and measure the efficacy of this approach on patient satisfaction with their care, patient activation, quality of life and use of healthcare resources.
ID: NCT03216109
Sponsor: Palo Alto Veterans Institute for Research
Location: VA Palo Alto Health Care System, California