User login
Mindfulness on Par With Drugs for Chronic Migraine in Patients Who’ve Overused Medication
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
AT THE AAN 2016 ANNUAL MEETING
Mindfulness on par with drugs for chronic migraine in patients who’ve overused medication
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
VANCOUVER – Mindfulness works as well as pharmacotherapy for prevention in patients who have chronic migraine with medication overuse, suggest initial findings of a trial reported at the annual meeting of the American Academy of Neurology.
Results at 6 months showed that both pharmacotherapy and a 6-week mindfulness training program netted 40% to 50% reductions in migraine days and medication uses per month, with no significant differences between these approaches.
“The effects of mindfulness alone seemed to be comparable to those obtained for medication alone in this particular group of patients,” commented lead author Dr. Licia Grazzi, a neurologist with the Headache Center, Carlo Besta Neurological Institute and Foundation, in Milan.
“We didn’t assess it specifically, but patients commented that mindfulness [produced no] side effects. Patients adhered very well to the treatment; they came to the sessions, so they could follow the treatment in an adequate way,” she added.
Giving some background, Dr. Grazzi noted that chronic migraine is especially disabling and hard to treatment in the context of medication overuse. Conventional management entails a period of medication weaning, followed by reintroduction of medication at appropriate levels plus adjunctive therapies as needed.
“Researchers have not studied the effectiveness of behavioral treatment alone following withdrawal,” Dr. Grazzi commented. “In particular, mindfulness is an emerging technique that has been recognized as a useful treatment for pain, but up until now, no application has been made for migraine and chronic migraine in particular.”
On average, the 44 patients enrolled in the trial had migraine on about 20 days of each month and used medication on most of those days.
All patients underwent a 5-day medication withdrawal in a day hospital treatment program. They were then split evenly into two groups: tailored pharmacologic prophylaxis and mindfulness training.
The mindfulness training entailed six 45-minute weekly sessions of guided meditation practice, with instructions to practice at home at least 7 minutes daily. In addition, patients were encouraged to get regular aerobic exercise and advised about lifestyle modifications as indicated.
Six months later, the pharmacotherapy group and mindfulness group had significant and statistically indistinguishable reductions in the number of migraine days per month (41.8% vs. 45.2%) and the number of medication uses per month (38.3% vs. 51.7%).
Both groups had a slight rebound in these measures between 3 and 6 months, but the increase in days of medication use was less marked in the mindfulness group. “Our explanation for that is that this group of patients can manage better their pain, and so they can reduce the use of medication when they suffer from pain,” Dr. Grazzi commented.
The pharmacotherapy and mindfulness groups also had comparable improvements in scores on the Migraine Disability Assessment (MIDAS) questionnaire (52.1% vs. 36.3%), the Beck Depression Inventory (41.9% vs. 33.8%), and the 6-item Headache Impact Test (HIT-6) (6.7% vs. 8.5%). State anxiety and trait anxiety improved in both groups as well.
“Data collection is now ongoing to determine if, with longer follow-up, we have significant results,” commented Dr. Grazzi. “It’s very common that patients after withdrawal tend to get better immediately, but then they relapse fairly easily. So we have to control and follow patients for 1 year to be sure about our encouraging results.”
Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Mindfulness shows efficacy similar to that of pharmacologic prophylaxis in patients with chronic migraine complicated by medication overuse.
Major finding: The pharmacotherapy group and the mindfulness group had similar 6-month improvements in migraine days per month (41.8% vs. 45.2%) and medication uses per month (38.3% vs. 51.7%).
Data source: A single-center trial involving 44 patients having chronic migraine with medication overuse.
Disclosures: Dr. Grazzi disclosed that she is a consultant/advisor for Allergan and ElectroCore Medical.
ATTAIN: Peginterferon beta-1a remains efficacious for MS in long term
VANCOUVER – Peginterferon beta-1a has durable efficacy for the treatment of relapsing-remitting multiple sclerosis, according to data from the ATTAIN extension study of a phase III trial.
With dosing every 2 weeks, as approved by the Food and Drug Administration, the 36% reduction in adjusted annualized relapse rate seen with peginterferon beta-1a relative to placebo in the first year persisted out to at least 6 years, investigators reported at the annual meeting of the American Academy of Neurology.
Additionally, the respective 67% and 86% reductions in new T2 lesions and gadolinium-enhancing lesions on magnetic resonance imaging that were observed in the first year of treatment persisted out to at least 4 years.
“Clinical and imaging [benefits were] sustained over up to a 6-year period in the pivotal and extension studies,” lead investigator Damian Fiore, Pharm.D., global medical director at Biogen in Cambridge, Mass., summarized in the emerging science session where he presented the data.
As the drug was approved based on the first-year findings, these new, longer-term data are a welcome confirmation of those initial benefits, according to session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center at San Antonio.
“Despite the open-label design of this company-sponsored study, the results are very encouraging for patients with multiple sclerosis as the analysis reveals year-over-year results with sustained efficacy,” he said in comments provided by email.
The pivotal, 2-year ADVANCE randomized trial (Efficacy and Safety Study of Peginterferon Beta-1a in Participants With Relapsing Multiple Sclerosis) began with a year of placebo in some patients and compared dosing strategies of peginterferon beta-1a (Plegridy).
“The ADVANCE trial was the largest trial that’s been done with interferon, at 1,500 patients,” Dr. Fiore commented. “One other important thing to note is that our colleagues on the interferon-alpha side have been using pegylation for quite a while, but this represents the first use of pegylation in MS therapy.”
Main results were published 3 years ago (Lancet Neurol. 2014;13:657-65) and led to approval of peginterferon beta-1a by the Food and Drug Administration for this indication shortly thereafter.
A total of 730 patients who started active treatment in the first year of the trial continued treatment on the ATTAIN extension study (Long-Term Safety and Efficacy Study of Peginterferon Beta-1a). The investigators reported data for the 376 patients given the drug every 2 weeks, analyzed after all had completed at least 96 weeks on the extension study.
Results for the intention-to-treat population showed that the adjusted annualized relapse rate ranged from 0.055 to 0.241 with peginterferon beta-1a in years 1 through 6 of treatment, compared with 0.418 with placebo in year 1.
The pattern was similar for the mean number of new T1 hypointense lesions (0.7-1.7 vs. 3.8 with placebo), new or newly enlarging T2 lesions (1.9-3.9 vs. 10.9), and gadolinium-enhancing lesions (0.2-0.3 vs. 1.4) during the first 4 years of treatment.
Dr. Fiore disclosed that he is an employee and stockholder of Biogen, which sponsored the trial.
VANCOUVER – Peginterferon beta-1a has durable efficacy for the treatment of relapsing-remitting multiple sclerosis, according to data from the ATTAIN extension study of a phase III trial.
With dosing every 2 weeks, as approved by the Food and Drug Administration, the 36% reduction in adjusted annualized relapse rate seen with peginterferon beta-1a relative to placebo in the first year persisted out to at least 6 years, investigators reported at the annual meeting of the American Academy of Neurology.
Additionally, the respective 67% and 86% reductions in new T2 lesions and gadolinium-enhancing lesions on magnetic resonance imaging that were observed in the first year of treatment persisted out to at least 4 years.
“Clinical and imaging [benefits were] sustained over up to a 6-year period in the pivotal and extension studies,” lead investigator Damian Fiore, Pharm.D., global medical director at Biogen in Cambridge, Mass., summarized in the emerging science session where he presented the data.
As the drug was approved based on the first-year findings, these new, longer-term data are a welcome confirmation of those initial benefits, according to session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center at San Antonio.
“Despite the open-label design of this company-sponsored study, the results are very encouraging for patients with multiple sclerosis as the analysis reveals year-over-year results with sustained efficacy,” he said in comments provided by email.
The pivotal, 2-year ADVANCE randomized trial (Efficacy and Safety Study of Peginterferon Beta-1a in Participants With Relapsing Multiple Sclerosis) began with a year of placebo in some patients and compared dosing strategies of peginterferon beta-1a (Plegridy).
“The ADVANCE trial was the largest trial that’s been done with interferon, at 1,500 patients,” Dr. Fiore commented. “One other important thing to note is that our colleagues on the interferon-alpha side have been using pegylation for quite a while, but this represents the first use of pegylation in MS therapy.”
Main results were published 3 years ago (Lancet Neurol. 2014;13:657-65) and led to approval of peginterferon beta-1a by the Food and Drug Administration for this indication shortly thereafter.
A total of 730 patients who started active treatment in the first year of the trial continued treatment on the ATTAIN extension study (Long-Term Safety and Efficacy Study of Peginterferon Beta-1a). The investigators reported data for the 376 patients given the drug every 2 weeks, analyzed after all had completed at least 96 weeks on the extension study.
Results for the intention-to-treat population showed that the adjusted annualized relapse rate ranged from 0.055 to 0.241 with peginterferon beta-1a in years 1 through 6 of treatment, compared with 0.418 with placebo in year 1.
The pattern was similar for the mean number of new T1 hypointense lesions (0.7-1.7 vs. 3.8 with placebo), new or newly enlarging T2 lesions (1.9-3.9 vs. 10.9), and gadolinium-enhancing lesions (0.2-0.3 vs. 1.4) during the first 4 years of treatment.
Dr. Fiore disclosed that he is an employee and stockholder of Biogen, which sponsored the trial.
VANCOUVER – Peginterferon beta-1a has durable efficacy for the treatment of relapsing-remitting multiple sclerosis, according to data from the ATTAIN extension study of a phase III trial.
With dosing every 2 weeks, as approved by the Food and Drug Administration, the 36% reduction in adjusted annualized relapse rate seen with peginterferon beta-1a relative to placebo in the first year persisted out to at least 6 years, investigators reported at the annual meeting of the American Academy of Neurology.
Additionally, the respective 67% and 86% reductions in new T2 lesions and gadolinium-enhancing lesions on magnetic resonance imaging that were observed in the first year of treatment persisted out to at least 4 years.
“Clinical and imaging [benefits were] sustained over up to a 6-year period in the pivotal and extension studies,” lead investigator Damian Fiore, Pharm.D., global medical director at Biogen in Cambridge, Mass., summarized in the emerging science session where he presented the data.
As the drug was approved based on the first-year findings, these new, longer-term data are a welcome confirmation of those initial benefits, according to session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center at San Antonio.
“Despite the open-label design of this company-sponsored study, the results are very encouraging for patients with multiple sclerosis as the analysis reveals year-over-year results with sustained efficacy,” he said in comments provided by email.
The pivotal, 2-year ADVANCE randomized trial (Efficacy and Safety Study of Peginterferon Beta-1a in Participants With Relapsing Multiple Sclerosis) began with a year of placebo in some patients and compared dosing strategies of peginterferon beta-1a (Plegridy).
“The ADVANCE trial was the largest trial that’s been done with interferon, at 1,500 patients,” Dr. Fiore commented. “One other important thing to note is that our colleagues on the interferon-alpha side have been using pegylation for quite a while, but this represents the first use of pegylation in MS therapy.”
Main results were published 3 years ago (Lancet Neurol. 2014;13:657-65) and led to approval of peginterferon beta-1a by the Food and Drug Administration for this indication shortly thereafter.
A total of 730 patients who started active treatment in the first year of the trial continued treatment on the ATTAIN extension study (Long-Term Safety and Efficacy Study of Peginterferon Beta-1a). The investigators reported data for the 376 patients given the drug every 2 weeks, analyzed after all had completed at least 96 weeks on the extension study.
Results for the intention-to-treat population showed that the adjusted annualized relapse rate ranged from 0.055 to 0.241 with peginterferon beta-1a in years 1 through 6 of treatment, compared with 0.418 with placebo in year 1.
The pattern was similar for the mean number of new T1 hypointense lesions (0.7-1.7 vs. 3.8 with placebo), new or newly enlarging T2 lesions (1.9-3.9 vs. 10.9), and gadolinium-enhancing lesions (0.2-0.3 vs. 1.4) during the first 4 years of treatment.
Dr. Fiore disclosed that he is an employee and stockholder of Biogen, which sponsored the trial.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Peginterferon beta-1a has sustained efficacy in treating relapsing-remitting multiple sclerosis for at least 6 years.
Major finding: The more than one-third reduction in annualized relapse rate seen in the first year of treatment persisted out to 6 years.
Data source: An extension study of a phase III trial among 730 patients with relapsing-remitting multiple sclerosis (ATTAIN study).
Disclosures: Dr. Fiore disclosed that he is an employee and stockholder of Biogen. The trial was sponsored by Biogen.
Doppler Ultrasound Headset Performs Well at Spotting Sports-related Concussion
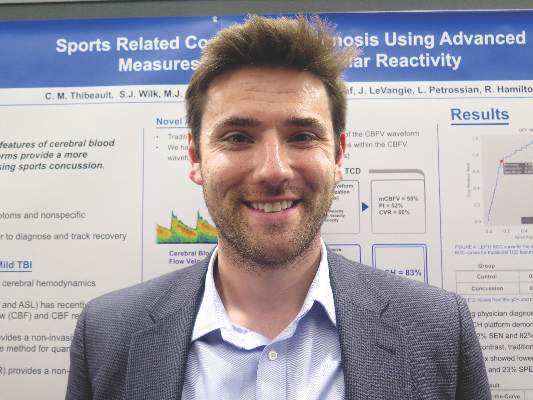
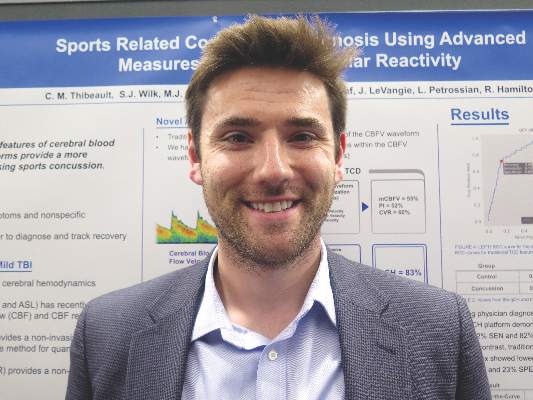
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
AT THE AAN 2016 ANNUAL MEETING
Doppler ultrasound headset performs well at spotting sports-related concussion
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Advanced transcranial Doppler analysis may improve identification of athletes with concussion at the point of care.
Major finding: For differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%.
Data source: A cohort study of 69 concussed and 169 nonconcussed high school athletes.
Disclosures: Dr. Hamilton disclosed that he is a cofounder and chief science officer of Neural Analytics. The study was supported by the National Institutes of Health and the National Science Foundation.
Scans show high prevalence of TBI among symptomatic retired NFL players
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
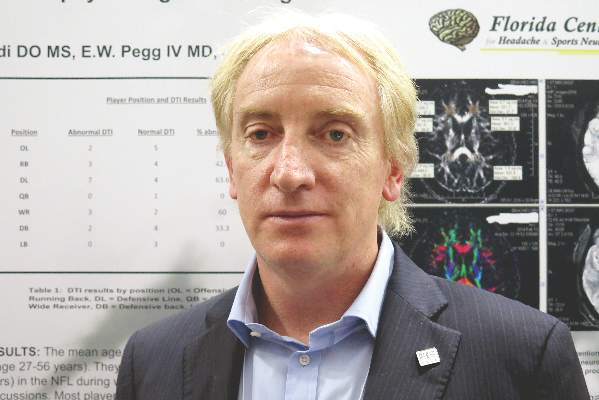
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: MRI findings suggest that traumatic brain injury is prevalent among symptomatic retired NFL players.
Major finding: Overall, 43% of the players had abnormal diffusion tensor MRI results and 30% had evidence of traumatic axonal injury on conventional MRI.
Data source: A prospective cohort study of 40 retired NFL players who sought care for neurocognitive symptoms.
Disclosures: Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
Once-daily eslicarbazepine equals twice-daily carbamazepine for controlling partial-onset seizures
VANCOUVER – Once-daily eslicarbazepine may offer a more convenient option for controlling newly diagnosed partial-onset seizures, suggest findings of a phase III trial reported at the annual meeting of the American Academy of Neurology.
The study of 815 adult patients found that 71.1% of those treated with once-daily eslicarbazepine as monotherapy were seizure free for at least 6 months, compared with 75.6% of those treated with twice-daily controlled-release carbamazepine as monotherapy. The difference fell within the predefined margin for noninferiority.
Additionally, the safety profile of eslicarbazepine was at least as good as that of carbamazepine, and there were no new or unexpected adverse events, relative to those seen in trials in which the former has been used as adjunctive therapy.
“Eslicarbazepine has the same efficacy, has a little bit better safety, and it can be taken once a day,” coauthor Dr. Pedro André Kowacs commented in an interview. “I have been working with it for 12 years. It’s a very, very good drug.”
“There are few antiepileptic drugs that can be taken once a day,” he added. “We know that a more simple schedule enhances adherence of the patient, compliance of the patient to therapy.”
Also, patients can choose when to take eslicarbazepine, according to Dr. Kowacs, who is a neurologist at the Instituto de Neurologia de Curitiba in Brazil. “You can take it in the morning or you can take it at night. It doesn’t matter,” he elaborated. “This is in contrast to, say, phenobarbital. If you take it in the morning, perhaps you are going to sleep all day long. And if you take three tabs of Dilantin (phenytoin), probably you get a little bit dizzy.”
In the trial, patients were randomized evenly to once-daily eslicarbazepine (brand name Aptiom) or twice-daily controlled-release carbamazepine (brand name Tegretol XR), each as monotherapy. Eslicarbazepine is currently approved by the U.S. Food and Drug Administration for the treatment of partial-onset seizures as monotherapy or adjunctive therapy.
In both groups, the patients were treated according to a three-step dose-level design, with upward titration of dose if they experienced seizures. However, the majority in each group – 67.6% for eslicarbazepine and 76.9% for carbamazepine – remained at the lowest-dose level studied (800 mg once daily and 200 mg twice daily, respectively).
The primary endpoint was the proportion of patients in the per-protocol population who were seizure free for the entire 26-week evaluation period at the last received dose level.
Overall, that proportion was 71.1% in the eslicarbazepine group and 75.6% in the carbamazepine group, Dr. Kowacs reported in an Emerging Science session. The absolute difference of –4.28% and the lower bound of the 95% confidence interval of –10.30% fell within the predefined noninferiority margin of –12%.
The 1-year rate of freedom from seizures was 64.7% in the eslicarbazepine group and 70.3% in the carbamazepine group. The absolute difference of –5.46% and the lower bound of the 95% confidence interval of –11.88% again fell within the noninferiority margin.
Patients in the eslicarbazepine tended to have a lower rate of treatment-emergent adverse events possibly related to the drug, compared with counterparts in the carbamazepine group (41.1% vs. 49.5%). The most common were dizziness and headache.
“The [gamma glutamyl transferase level] was increased in more patients taking carbamazepine,” Dr. Kowacs noted, with a rate of 12.4% versus 2.7% with eslicarbazepine. “Hyponatremia occurred in both groups, but no patient was symptomatic.”
There were two deaths each in the eslicarbazepine group (from glioblastoma and cardiac arrest) and the carbamazepine group (from suicide and lung cancer).
Dr. Kowacs disclosed that he has received personal compensation for activities with Bial, Abbott Laboratories, GlaxoSmithKline, and Cyberonics. The trial was sponsored by Bial-Portela & Cª SA.
VANCOUVER – Once-daily eslicarbazepine may offer a more convenient option for controlling newly diagnosed partial-onset seizures, suggest findings of a phase III trial reported at the annual meeting of the American Academy of Neurology.
The study of 815 adult patients found that 71.1% of those treated with once-daily eslicarbazepine as monotherapy were seizure free for at least 6 months, compared with 75.6% of those treated with twice-daily controlled-release carbamazepine as monotherapy. The difference fell within the predefined margin for noninferiority.
Additionally, the safety profile of eslicarbazepine was at least as good as that of carbamazepine, and there were no new or unexpected adverse events, relative to those seen in trials in which the former has been used as adjunctive therapy.
“Eslicarbazepine has the same efficacy, has a little bit better safety, and it can be taken once a day,” coauthor Dr. Pedro André Kowacs commented in an interview. “I have been working with it for 12 years. It’s a very, very good drug.”
“There are few antiepileptic drugs that can be taken once a day,” he added. “We know that a more simple schedule enhances adherence of the patient, compliance of the patient to therapy.”
Also, patients can choose when to take eslicarbazepine, according to Dr. Kowacs, who is a neurologist at the Instituto de Neurologia de Curitiba in Brazil. “You can take it in the morning or you can take it at night. It doesn’t matter,” he elaborated. “This is in contrast to, say, phenobarbital. If you take it in the morning, perhaps you are going to sleep all day long. And if you take three tabs of Dilantin (phenytoin), probably you get a little bit dizzy.”
In the trial, patients were randomized evenly to once-daily eslicarbazepine (brand name Aptiom) or twice-daily controlled-release carbamazepine (brand name Tegretol XR), each as monotherapy. Eslicarbazepine is currently approved by the U.S. Food and Drug Administration for the treatment of partial-onset seizures as monotherapy or adjunctive therapy.
In both groups, the patients were treated according to a three-step dose-level design, with upward titration of dose if they experienced seizures. However, the majority in each group – 67.6% for eslicarbazepine and 76.9% for carbamazepine – remained at the lowest-dose level studied (800 mg once daily and 200 mg twice daily, respectively).
The primary endpoint was the proportion of patients in the per-protocol population who were seizure free for the entire 26-week evaluation period at the last received dose level.
Overall, that proportion was 71.1% in the eslicarbazepine group and 75.6% in the carbamazepine group, Dr. Kowacs reported in an Emerging Science session. The absolute difference of –4.28% and the lower bound of the 95% confidence interval of –10.30% fell within the predefined noninferiority margin of –12%.
The 1-year rate of freedom from seizures was 64.7% in the eslicarbazepine group and 70.3% in the carbamazepine group. The absolute difference of –5.46% and the lower bound of the 95% confidence interval of –11.88% again fell within the noninferiority margin.
Patients in the eslicarbazepine tended to have a lower rate of treatment-emergent adverse events possibly related to the drug, compared with counterparts in the carbamazepine group (41.1% vs. 49.5%). The most common were dizziness and headache.
“The [gamma glutamyl transferase level] was increased in more patients taking carbamazepine,” Dr. Kowacs noted, with a rate of 12.4% versus 2.7% with eslicarbazepine. “Hyponatremia occurred in both groups, but no patient was symptomatic.”
There were two deaths each in the eslicarbazepine group (from glioblastoma and cardiac arrest) and the carbamazepine group (from suicide and lung cancer).
Dr. Kowacs disclosed that he has received personal compensation for activities with Bial, Abbott Laboratories, GlaxoSmithKline, and Cyberonics. The trial was sponsored by Bial-Portela & Cª SA.
VANCOUVER – Once-daily eslicarbazepine may offer a more convenient option for controlling newly diagnosed partial-onset seizures, suggest findings of a phase III trial reported at the annual meeting of the American Academy of Neurology.
The study of 815 adult patients found that 71.1% of those treated with once-daily eslicarbazepine as monotherapy were seizure free for at least 6 months, compared with 75.6% of those treated with twice-daily controlled-release carbamazepine as monotherapy. The difference fell within the predefined margin for noninferiority.
Additionally, the safety profile of eslicarbazepine was at least as good as that of carbamazepine, and there were no new or unexpected adverse events, relative to those seen in trials in which the former has been used as adjunctive therapy.
“Eslicarbazepine has the same efficacy, has a little bit better safety, and it can be taken once a day,” coauthor Dr. Pedro André Kowacs commented in an interview. “I have been working with it for 12 years. It’s a very, very good drug.”
“There are few antiepileptic drugs that can be taken once a day,” he added. “We know that a more simple schedule enhances adherence of the patient, compliance of the patient to therapy.”
Also, patients can choose when to take eslicarbazepine, according to Dr. Kowacs, who is a neurologist at the Instituto de Neurologia de Curitiba in Brazil. “You can take it in the morning or you can take it at night. It doesn’t matter,” he elaborated. “This is in contrast to, say, phenobarbital. If you take it in the morning, perhaps you are going to sleep all day long. And if you take three tabs of Dilantin (phenytoin), probably you get a little bit dizzy.”
In the trial, patients were randomized evenly to once-daily eslicarbazepine (brand name Aptiom) or twice-daily controlled-release carbamazepine (brand name Tegretol XR), each as monotherapy. Eslicarbazepine is currently approved by the U.S. Food and Drug Administration for the treatment of partial-onset seizures as monotherapy or adjunctive therapy.
In both groups, the patients were treated according to a three-step dose-level design, with upward titration of dose if they experienced seizures. However, the majority in each group – 67.6% for eslicarbazepine and 76.9% for carbamazepine – remained at the lowest-dose level studied (800 mg once daily and 200 mg twice daily, respectively).
The primary endpoint was the proportion of patients in the per-protocol population who were seizure free for the entire 26-week evaluation period at the last received dose level.
Overall, that proportion was 71.1% in the eslicarbazepine group and 75.6% in the carbamazepine group, Dr. Kowacs reported in an Emerging Science session. The absolute difference of –4.28% and the lower bound of the 95% confidence interval of –10.30% fell within the predefined noninferiority margin of –12%.
The 1-year rate of freedom from seizures was 64.7% in the eslicarbazepine group and 70.3% in the carbamazepine group. The absolute difference of –5.46% and the lower bound of the 95% confidence interval of –11.88% again fell within the noninferiority margin.
Patients in the eslicarbazepine tended to have a lower rate of treatment-emergent adverse events possibly related to the drug, compared with counterparts in the carbamazepine group (41.1% vs. 49.5%). The most common were dizziness and headache.
“The [gamma glutamyl transferase level] was increased in more patients taking carbamazepine,” Dr. Kowacs noted, with a rate of 12.4% versus 2.7% with eslicarbazepine. “Hyponatremia occurred in both groups, but no patient was symptomatic.”
There were two deaths each in the eslicarbazepine group (from glioblastoma and cardiac arrest) and the carbamazepine group (from suicide and lung cancer).
Dr. Kowacs disclosed that he has received personal compensation for activities with Bial, Abbott Laboratories, GlaxoSmithKline, and Cyberonics. The trial was sponsored by Bial-Portela & Cª SA.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Once-daily eslicarbazepine has similar safety and efficacy as twice-daily carbamazepine in patients with partial-onset seizures.
Major finding: The proportion of patients who were seizure free for at least 6 months at the last evaluated dose was 71.1% with once-daily eslicarbazepine and 75.6% with twice-daily controlled-release carbamazepine, a noninferior difference.
Data source: A phase III, randomized, controlled trial among 815 adults with newly diagnosed partial-onset seizures.
Disclosures: Dr. Kowacs disclosed that he has received personal compensation for activities with Bial, Abbott Laboratories, GlaxoSmithKline, and Cyberonics. The trial was sponsored by Bial-Portela & Cª SA.
New data help guide the stopping of disease-modifying drugs in MS
VANCOUVER – Certain patient and disease characteristics may help guide decisions about starting and stopping therapy in progressive multiple sclerosis, according to a pair of longitudinal cohort studies reported at the annual meeting of the American Academy of Neurology.
In a cohort of patients transitioning from relapsing-remitting to progressive multiple sclerosis (MS), the age at onset of progression predicted the likelihood of subsequent relapses. The absolute lifetime risk ranged from 18% for patients younger than 35 years at the time to just 5% for those aged 55 years or older at the time.
And in a cohort of patients with secondary progressive MS, the annual rate of clinical relapse fell in the third year after immunomodulator discontinuation. Overall, 35% had a clinical relapse or radiologic disease activity. Patients had a lower risk of this outcome if they had greater disability at the time of discontinuation or if they had not had any radiologic disease activity in the antecedent years.
“These studies address a very important question, because not many people talk about stopping drugs,” commented session comoderator Helen Tremlett, Ph.D., of the division of neurology at the University of British Columbia, Vancouver, and the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis. “I would like to see some of these findings validated in other cohorts. But I did like the questions they are asking.”
Age, risk of postprogression relapse
The first study focused on ongoing relapses in patients transitioning to progressive MS. “We have recently shown that these relapses indeed matter. People who continue to relapse after progressive MS onset develop a need for a cane 2 years earlier than those who don’t” continue to have relapses, explained senior author Dr. Orhun H. Kantarci of the department of neurology at the Mayo Clinic in Rochester, Minn. Therefore, continuation or initiation of disease-modifying drugs (DMDs) during this period of overlap may be beneficial.
He and his colleagues followed 946 patients with MS from a clinic- and population-based cohort, assessing the age at various disease landmarks.
Results showed the mean age at first relapse was 33 years, the mean age at the onset of progressive MS was 45 years, and the mean age at last relapse (whether it occurred before or after the onset of progressive disease) was 43 years.
The 95% overlap age range for age at first relapse and age at onset of progressive MS was 27-46 years, Dr. Kantarci reported. Therefore, DMDs would be expected to have a some impact during those years.
Further analyses showed that if the age of progressive MS onset was before 35 years, 35-44 years, 45-54 years, and 55 years or older, the absolute lifetime risk of relapse after progressive MS onset was 18%, 17%, 13%, and 5%, respectively. The corresponding last predicted relapse for these groups was before age 50, age 60, age 70, and age 70.
Taken together, the data suggest that in patients transitioning to progressive MS, initiation or continuation of a DMD is most likely to be beneficial if the patient is younger than 35, with some benefit, albeit less, up to the age of 54, according to Dr. Kantarci.
“But above 55, if a person has never been on a DMD, it is unlikely to be recommended, because I don’t expect it to do anything from the data we have,” he said. Furthermore, “DMD stopping can be offered to these patients. If a person is on a DMD and they are asking, ‘Can I stop it? I have been stable,’ and they are above age 55, it can be considered.”
The investigators are performing additional analyses of the data to assess the impact of DMDs on disease course, including the influence of initial and maintenance treatment choices, and factors that prompt physicians to switch treatments.
“What we haven’t done and will not be possible with this data ... the most interesting question is the impact of subclinical MRI disease, which is a different question,” Dr. Kantarci concluded. “And ultimately, we will have to have an actual stopping experiment and [assess] outcomes, which is an ongoing major planned effort.”
Outcomes after stopping immunomodulators
The second study assessed clinical and radiologic outcomes after discontinuation of immunomodulatory therapy in patients with secondary progressive MS.
“We have more and more patients [with secondary progressive disease] treated for several years, yet the natural history of the disease is less and less relapse, and progression of disability,” commented first author Dr. Julien Bonenfant, a neurologist at the Rennes University Hospital in France. Thus, the benefit of continuing treatment is unclear, especially given its cost and side effects.
He and his colleagues studied 106 consecutive patients with secondary progressive MS who had been on immunomodulators for at least 6 months, were taken off the immunomodulators, and were followed for a mean of 5 years.
Results showed that 16% of the patients had a clinical relapse after discontinuation, nearly all within the first 3 years. The annualized rate of clinical relapse actually fell from 0.13 in the 3 years before discontinuation to 0.07 in the 3 years afterward.
Overall, 35% of patients had either a clinical relapse and/or new contrast enhancement on MRI. Again, most of these events occurred within the first 3 years of discontinuation.
Patients had a lower risk of this outcome after treatment discontinuation if they had an Expanded Disability Status Scale (EDSS) score of 6 or greater at the time of discontinuation (hazard ratio, 0.4) and if they had not had any gadolinium enhancement on MRI in the 3 years before treatment discontinuation (HR, 0.4).
“Disease activity remained low after treatment withdrawal. We found no rebound of relapse rate in our population,” Dr. Bonenfant summarized. “There was no consequence on the slope of disability progression,” nor on the finding of enhancement on MRI alone.
Thirty patients were restarted on immunomodulators, about half of them solely because of MRI findings, he noted. This raises “the controversial question, is it relevant or not to resume treatment in these patients?”
“This study suggests that immunomodulatory treatment withdrawal seems reasonable for patients with advanced secondary progressive MS, especially with an EDSS of 6 or greater and no focal inflammatory disease [clinical or radiologic] at least in the past 3 years,” Dr. Bonenfant maintained. “It shows the importance of MRI monitoring to define the patients who are still in a focal immunoreactive state.”
“The results are far from being definitive, and further prospective studies are needed to provide evidence-based recommendations for clinical practice,” he concluded.
Dr. Kantarci disclosed that he has given scientific presentations at meetings supported by Novartis Pharmaceuticals and has presented as an invited speaker for Biogen but has received no personal compensation from either company. Dr. Bonenfant disclosed that he had no relevant conflicts of interest.
VANCOUVER – Certain patient and disease characteristics may help guide decisions about starting and stopping therapy in progressive multiple sclerosis, according to a pair of longitudinal cohort studies reported at the annual meeting of the American Academy of Neurology.
In a cohort of patients transitioning from relapsing-remitting to progressive multiple sclerosis (MS), the age at onset of progression predicted the likelihood of subsequent relapses. The absolute lifetime risk ranged from 18% for patients younger than 35 years at the time to just 5% for those aged 55 years or older at the time.
And in a cohort of patients with secondary progressive MS, the annual rate of clinical relapse fell in the third year after immunomodulator discontinuation. Overall, 35% had a clinical relapse or radiologic disease activity. Patients had a lower risk of this outcome if they had greater disability at the time of discontinuation or if they had not had any radiologic disease activity in the antecedent years.
“These studies address a very important question, because not many people talk about stopping drugs,” commented session comoderator Helen Tremlett, Ph.D., of the division of neurology at the University of British Columbia, Vancouver, and the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis. “I would like to see some of these findings validated in other cohorts. But I did like the questions they are asking.”
Age, risk of postprogression relapse
The first study focused on ongoing relapses in patients transitioning to progressive MS. “We have recently shown that these relapses indeed matter. People who continue to relapse after progressive MS onset develop a need for a cane 2 years earlier than those who don’t” continue to have relapses, explained senior author Dr. Orhun H. Kantarci of the department of neurology at the Mayo Clinic in Rochester, Minn. Therefore, continuation or initiation of disease-modifying drugs (DMDs) during this period of overlap may be beneficial.
He and his colleagues followed 946 patients with MS from a clinic- and population-based cohort, assessing the age at various disease landmarks.
Results showed the mean age at first relapse was 33 years, the mean age at the onset of progressive MS was 45 years, and the mean age at last relapse (whether it occurred before or after the onset of progressive disease) was 43 years.
The 95% overlap age range for age at first relapse and age at onset of progressive MS was 27-46 years, Dr. Kantarci reported. Therefore, DMDs would be expected to have a some impact during those years.
Further analyses showed that if the age of progressive MS onset was before 35 years, 35-44 years, 45-54 years, and 55 years or older, the absolute lifetime risk of relapse after progressive MS onset was 18%, 17%, 13%, and 5%, respectively. The corresponding last predicted relapse for these groups was before age 50, age 60, age 70, and age 70.
Taken together, the data suggest that in patients transitioning to progressive MS, initiation or continuation of a DMD is most likely to be beneficial if the patient is younger than 35, with some benefit, albeit less, up to the age of 54, according to Dr. Kantarci.
“But above 55, if a person has never been on a DMD, it is unlikely to be recommended, because I don’t expect it to do anything from the data we have,” he said. Furthermore, “DMD stopping can be offered to these patients. If a person is on a DMD and they are asking, ‘Can I stop it? I have been stable,’ and they are above age 55, it can be considered.”
The investigators are performing additional analyses of the data to assess the impact of DMDs on disease course, including the influence of initial and maintenance treatment choices, and factors that prompt physicians to switch treatments.
“What we haven’t done and will not be possible with this data ... the most interesting question is the impact of subclinical MRI disease, which is a different question,” Dr. Kantarci concluded. “And ultimately, we will have to have an actual stopping experiment and [assess] outcomes, which is an ongoing major planned effort.”
Outcomes after stopping immunomodulators
The second study assessed clinical and radiologic outcomes after discontinuation of immunomodulatory therapy in patients with secondary progressive MS.
“We have more and more patients [with secondary progressive disease] treated for several years, yet the natural history of the disease is less and less relapse, and progression of disability,” commented first author Dr. Julien Bonenfant, a neurologist at the Rennes University Hospital in France. Thus, the benefit of continuing treatment is unclear, especially given its cost and side effects.
He and his colleagues studied 106 consecutive patients with secondary progressive MS who had been on immunomodulators for at least 6 months, were taken off the immunomodulators, and were followed for a mean of 5 years.
Results showed that 16% of the patients had a clinical relapse after discontinuation, nearly all within the first 3 years. The annualized rate of clinical relapse actually fell from 0.13 in the 3 years before discontinuation to 0.07 in the 3 years afterward.
Overall, 35% of patients had either a clinical relapse and/or new contrast enhancement on MRI. Again, most of these events occurred within the first 3 years of discontinuation.
Patients had a lower risk of this outcome after treatment discontinuation if they had an Expanded Disability Status Scale (EDSS) score of 6 or greater at the time of discontinuation (hazard ratio, 0.4) and if they had not had any gadolinium enhancement on MRI in the 3 years before treatment discontinuation (HR, 0.4).
“Disease activity remained low after treatment withdrawal. We found no rebound of relapse rate in our population,” Dr. Bonenfant summarized. “There was no consequence on the slope of disability progression,” nor on the finding of enhancement on MRI alone.
Thirty patients were restarted on immunomodulators, about half of them solely because of MRI findings, he noted. This raises “the controversial question, is it relevant or not to resume treatment in these patients?”
“This study suggests that immunomodulatory treatment withdrawal seems reasonable for patients with advanced secondary progressive MS, especially with an EDSS of 6 or greater and no focal inflammatory disease [clinical or radiologic] at least in the past 3 years,” Dr. Bonenfant maintained. “It shows the importance of MRI monitoring to define the patients who are still in a focal immunoreactive state.”
“The results are far from being definitive, and further prospective studies are needed to provide evidence-based recommendations for clinical practice,” he concluded.
Dr. Kantarci disclosed that he has given scientific presentations at meetings supported by Novartis Pharmaceuticals and has presented as an invited speaker for Biogen but has received no personal compensation from either company. Dr. Bonenfant disclosed that he had no relevant conflicts of interest.
VANCOUVER – Certain patient and disease characteristics may help guide decisions about starting and stopping therapy in progressive multiple sclerosis, according to a pair of longitudinal cohort studies reported at the annual meeting of the American Academy of Neurology.
In a cohort of patients transitioning from relapsing-remitting to progressive multiple sclerosis (MS), the age at onset of progression predicted the likelihood of subsequent relapses. The absolute lifetime risk ranged from 18% for patients younger than 35 years at the time to just 5% for those aged 55 years or older at the time.
And in a cohort of patients with secondary progressive MS, the annual rate of clinical relapse fell in the third year after immunomodulator discontinuation. Overall, 35% had a clinical relapse or radiologic disease activity. Patients had a lower risk of this outcome if they had greater disability at the time of discontinuation or if they had not had any radiologic disease activity in the antecedent years.
“These studies address a very important question, because not many people talk about stopping drugs,” commented session comoderator Helen Tremlett, Ph.D., of the division of neurology at the University of British Columbia, Vancouver, and the Canada Research Chair in Neuroepidemiology and Multiple Sclerosis. “I would like to see some of these findings validated in other cohorts. But I did like the questions they are asking.”
Age, risk of postprogression relapse
The first study focused on ongoing relapses in patients transitioning to progressive MS. “We have recently shown that these relapses indeed matter. People who continue to relapse after progressive MS onset develop a need for a cane 2 years earlier than those who don’t” continue to have relapses, explained senior author Dr. Orhun H. Kantarci of the department of neurology at the Mayo Clinic in Rochester, Minn. Therefore, continuation or initiation of disease-modifying drugs (DMDs) during this period of overlap may be beneficial.
He and his colleagues followed 946 patients with MS from a clinic- and population-based cohort, assessing the age at various disease landmarks.
Results showed the mean age at first relapse was 33 years, the mean age at the onset of progressive MS was 45 years, and the mean age at last relapse (whether it occurred before or after the onset of progressive disease) was 43 years.
The 95% overlap age range for age at first relapse and age at onset of progressive MS was 27-46 years, Dr. Kantarci reported. Therefore, DMDs would be expected to have a some impact during those years.
Further analyses showed that if the age of progressive MS onset was before 35 years, 35-44 years, 45-54 years, and 55 years or older, the absolute lifetime risk of relapse after progressive MS onset was 18%, 17%, 13%, and 5%, respectively. The corresponding last predicted relapse for these groups was before age 50, age 60, age 70, and age 70.
Taken together, the data suggest that in patients transitioning to progressive MS, initiation or continuation of a DMD is most likely to be beneficial if the patient is younger than 35, with some benefit, albeit less, up to the age of 54, according to Dr. Kantarci.
“But above 55, if a person has never been on a DMD, it is unlikely to be recommended, because I don’t expect it to do anything from the data we have,” he said. Furthermore, “DMD stopping can be offered to these patients. If a person is on a DMD and they are asking, ‘Can I stop it? I have been stable,’ and they are above age 55, it can be considered.”
The investigators are performing additional analyses of the data to assess the impact of DMDs on disease course, including the influence of initial and maintenance treatment choices, and factors that prompt physicians to switch treatments.
“What we haven’t done and will not be possible with this data ... the most interesting question is the impact of subclinical MRI disease, which is a different question,” Dr. Kantarci concluded. “And ultimately, we will have to have an actual stopping experiment and [assess] outcomes, which is an ongoing major planned effort.”
Outcomes after stopping immunomodulators
The second study assessed clinical and radiologic outcomes after discontinuation of immunomodulatory therapy in patients with secondary progressive MS.
“We have more and more patients [with secondary progressive disease] treated for several years, yet the natural history of the disease is less and less relapse, and progression of disability,” commented first author Dr. Julien Bonenfant, a neurologist at the Rennes University Hospital in France. Thus, the benefit of continuing treatment is unclear, especially given its cost and side effects.
He and his colleagues studied 106 consecutive patients with secondary progressive MS who had been on immunomodulators for at least 6 months, were taken off the immunomodulators, and were followed for a mean of 5 years.
Results showed that 16% of the patients had a clinical relapse after discontinuation, nearly all within the first 3 years. The annualized rate of clinical relapse actually fell from 0.13 in the 3 years before discontinuation to 0.07 in the 3 years afterward.
Overall, 35% of patients had either a clinical relapse and/or new contrast enhancement on MRI. Again, most of these events occurred within the first 3 years of discontinuation.
Patients had a lower risk of this outcome after treatment discontinuation if they had an Expanded Disability Status Scale (EDSS) score of 6 or greater at the time of discontinuation (hazard ratio, 0.4) and if they had not had any gadolinium enhancement on MRI in the 3 years before treatment discontinuation (HR, 0.4).
“Disease activity remained low after treatment withdrawal. We found no rebound of relapse rate in our population,” Dr. Bonenfant summarized. “There was no consequence on the slope of disability progression,” nor on the finding of enhancement on MRI alone.
Thirty patients were restarted on immunomodulators, about half of them solely because of MRI findings, he noted. This raises “the controversial question, is it relevant or not to resume treatment in these patients?”
“This study suggests that immunomodulatory treatment withdrawal seems reasonable for patients with advanced secondary progressive MS, especially with an EDSS of 6 or greater and no focal inflammatory disease [clinical or radiologic] at least in the past 3 years,” Dr. Bonenfant maintained. “It shows the importance of MRI monitoring to define the patients who are still in a focal immunoreactive state.”
“The results are far from being definitive, and further prospective studies are needed to provide evidence-based recommendations for clinical practice,” he concluded.
Dr. Kantarci disclosed that he has given scientific presentations at meetings supported by Novartis Pharmaceuticals and has presented as an invited speaker for Biogen but has received no personal compensation from either company. Dr. Bonenfant disclosed that he had no relevant conflicts of interest.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Patient and disease characteristics may help guide decisions about starting and stopping therapy in progressive MS.
Major finding: The absolute risk of symptomatic relapse after the onset of progressive disease fell with the age at this onset, from 18% for patients younger than 35 at that time to 5% in patients 55 and older. In patients with secondary progressive disease, the annualized clinical relapse rate was 0.13 in the 3 years before and 0.07 in the 3 years after immunomodulator discontinuation.
Data source: A pair of longitudinal cohort studies in 964 patients transitioning to progressive MS and 106 patients with secondary progressive MS.
Disclosures: Dr. Kantarci disclosed that he has given scientific presentations at meetings supported by Novartis Pharmaceuticals and has presented as an invited speaker for Biogen, but has received no personal compensation from either company. Dr. Bonenfant disclosed that he had no relevant conflicts of interest.
Anti-CGRP agent delivered durable episodic migraine prevention
VANCOUVER – An investigational antibody that targets the calcitonin gene-related peptide (CGRP) receptor may be efficacious and safe in the longer term for preventing episodic migraine, data from an ongoing extension of a phase II trial showed.
Among the 483 adults studied, the benefit seen with Amgen’s 334 antibody (AMG 334) at the highest dose at 12 weeks persisted out to a year, according to interim results reported at the annual meeting of the American Academy of Neurology.
At that time, patients on average were having roughly 5 fewer migraine days each month. Nearly two-thirds had experienced at least a halving of monthly migraine days, and nearly one-fifth had become totally migraine free. In addition, the antibody’s good safety profile also held up.
“These data strongly support the further investigation of AMG 334 as a potential preventive medication,” commented presenting author Dr. Stephen Silberstein, professor and director of the headache center at Thomas Jefferson University, Philadelphia.
“If you give a monoclonal antibody to someone with migraine and it takes away the associated features, then you just wind up with a headache day,” Dr. Silberstein noted. “But analysis clearly shows the reduction in migraine days with a concomitant reduction in headache days, showing you are not just removing the associated symptoms.”
It is too early to say whether the antibody is actually modifying the underlying disease, according to Dr. Silberstein. That will require a randomized withdrawal trial, assessing whether patients who stop treatment have a recurrence.
In any event, on the basis of the favorable findings from the phase II trial and its extension, a phase III trial, the Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention (STRIVE), is now underway.
Four antibodies targeting CGRP signaling are now being investigated for migraine prevention, but AMG 334 is the only one targeting the receptor, noted session attendee Dr. Denise E. Chou of the department of neurology at Columbia University and director of the Columbia University headache center, New York.
“Particularly striking is the almost 20% rate of 100% remission. That’s remarkable, because it’s unheard of for any of the current existing migraine therapies,” she noted. The antibody’s longer half-life (translating to greater convenience for patients) and good safety profile are also pluses.
“With the four different antibodies out there, it’s kind of a race,” Dr. Chou commented. “We’ll have to see which have the best efficacy rates and side effect profiles, and which are going to be the best in terms of patient compliance and dosing scheme.”
Compared with some other trials, the AMG 334 trial enrolled a population that had episodic migraine, which is relatively easier to treat, Dr. Chou noted.
“We know, in general, that chronic refractory patients tend to have lower response rates to therapies,” she said. “But it is nice that there are different studies looking at different populations, because that’s what we see, a very heterogeneous clinic population. To be able to reflect the real-world setting is important.”
Patients were eligible for the Amgen-sponsored trial if they had 4-14 migraine days and fewer than 15 headache days per month. They could have experienced failure of up to two previous prophylactic therapies. Rescue medications were allowed, but medication overuse was not.
The patients were randomized 2:2:2:3 to monthly subcutaneous injections of AMG 334 at doses of 7 mg, 21 mg, or 70 mg, or to placebo, on a double-blind basis.
At baseline, the patients had about 9 migraine days and 10 headache days each month so that migraine accounted for most of the total, Dr. Silberstein noted.
At 12 weeks, the change in monthly migraine days – the trial’s primary outcome – was greater with the 70-mg dose than with placebo, with a reduction of 3.40 days vs. 2.28 days each month (P = .021) (Lancet Neurol. 2016 Apr.;15[4]:382-90).
A post hoc analysis suggested that this difference was already significant merely 2 weeks into treatment, Dr. Silberstein reported. The reductions seen with the lower doses were not significant.
All patients were then switched to the 70-mg dose of AMG 334 on the open-label extension, which is planned to continue for up to 5 years. The results showed that both lower-dose groups and the placebo group quickly caught up to their peers on the higher dose when it came to the reduction in monthly migraine days.
“In contrast to both the topiramate and botulinum [toxin] studies, at 16 weeks, there was no difference between patients who had started on the higher dose of [AMG 334] or who were transferred over,” Dr. Silberstein commented. “If one does an individual analysis of patients who were before on placebo or the lower doses, each of them caught up, which suggests a difference in the [mechanism of] … botulinum toxin or topiramate – because with those drugs, they never caught up at the end of 6 months.”
At 52 weeks, the patients as a whole were having about 5 fewer migraine days each month, compared with baseline. Overall, 62.3% of patients had achieved a reduction of at least 50% in monthly migraine days; 38.4%, a reduction of at least 75%; and 18.5%, a reduction of 100%.
The pattern was similar when it came to monthly migraine-specific medication days, monthly headache days, and monthly migraine attacks, according to Dr. Silberstein.
“The safety and tolerability during the open-label phase were similar to that in the double-blind phase,” he noted. “One of the reasons this is important is that, unlike small molecules, the monoclonal antibodies are not metabolized in the liver, but are metabolized in the reticuloendothelial system. So, the concept of small-molecule toxicity does not exist.”
Amgen sponsored the trial. Dr. Silberstein disclosed that he receives consulting fees from Amgen.
VANCOUVER – An investigational antibody that targets the calcitonin gene-related peptide (CGRP) receptor may be efficacious and safe in the longer term for preventing episodic migraine, data from an ongoing extension of a phase II trial showed.
Among the 483 adults studied, the benefit seen with Amgen’s 334 antibody (AMG 334) at the highest dose at 12 weeks persisted out to a year, according to interim results reported at the annual meeting of the American Academy of Neurology.
At that time, patients on average were having roughly 5 fewer migraine days each month. Nearly two-thirds had experienced at least a halving of monthly migraine days, and nearly one-fifth had become totally migraine free. In addition, the antibody’s good safety profile also held up.
“These data strongly support the further investigation of AMG 334 as a potential preventive medication,” commented presenting author Dr. Stephen Silberstein, professor and director of the headache center at Thomas Jefferson University, Philadelphia.
“If you give a monoclonal antibody to someone with migraine and it takes away the associated features, then you just wind up with a headache day,” Dr. Silberstein noted. “But analysis clearly shows the reduction in migraine days with a concomitant reduction in headache days, showing you are not just removing the associated symptoms.”
It is too early to say whether the antibody is actually modifying the underlying disease, according to Dr. Silberstein. That will require a randomized withdrawal trial, assessing whether patients who stop treatment have a recurrence.
In any event, on the basis of the favorable findings from the phase II trial and its extension, a phase III trial, the Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention (STRIVE), is now underway.
Four antibodies targeting CGRP signaling are now being investigated for migraine prevention, but AMG 334 is the only one targeting the receptor, noted session attendee Dr. Denise E. Chou of the department of neurology at Columbia University and director of the Columbia University headache center, New York.
“Particularly striking is the almost 20% rate of 100% remission. That’s remarkable, because it’s unheard of for any of the current existing migraine therapies,” she noted. The antibody’s longer half-life (translating to greater convenience for patients) and good safety profile are also pluses.
“With the four different antibodies out there, it’s kind of a race,” Dr. Chou commented. “We’ll have to see which have the best efficacy rates and side effect profiles, and which are going to be the best in terms of patient compliance and dosing scheme.”
Compared with some other trials, the AMG 334 trial enrolled a population that had episodic migraine, which is relatively easier to treat, Dr. Chou noted.
“We know, in general, that chronic refractory patients tend to have lower response rates to therapies,” she said. “But it is nice that there are different studies looking at different populations, because that’s what we see, a very heterogeneous clinic population. To be able to reflect the real-world setting is important.”
Patients were eligible for the Amgen-sponsored trial if they had 4-14 migraine days and fewer than 15 headache days per month. They could have experienced failure of up to two previous prophylactic therapies. Rescue medications were allowed, but medication overuse was not.
The patients were randomized 2:2:2:3 to monthly subcutaneous injections of AMG 334 at doses of 7 mg, 21 mg, or 70 mg, or to placebo, on a double-blind basis.
At baseline, the patients had about 9 migraine days and 10 headache days each month so that migraine accounted for most of the total, Dr. Silberstein noted.
At 12 weeks, the change in monthly migraine days – the trial’s primary outcome – was greater with the 70-mg dose than with placebo, with a reduction of 3.40 days vs. 2.28 days each month (P = .021) (Lancet Neurol. 2016 Apr.;15[4]:382-90).
A post hoc analysis suggested that this difference was already significant merely 2 weeks into treatment, Dr. Silberstein reported. The reductions seen with the lower doses were not significant.
All patients were then switched to the 70-mg dose of AMG 334 on the open-label extension, which is planned to continue for up to 5 years. The results showed that both lower-dose groups and the placebo group quickly caught up to their peers on the higher dose when it came to the reduction in monthly migraine days.
“In contrast to both the topiramate and botulinum [toxin] studies, at 16 weeks, there was no difference between patients who had started on the higher dose of [AMG 334] or who were transferred over,” Dr. Silberstein commented. “If one does an individual analysis of patients who were before on placebo or the lower doses, each of them caught up, which suggests a difference in the [mechanism of] … botulinum toxin or topiramate – because with those drugs, they never caught up at the end of 6 months.”
At 52 weeks, the patients as a whole were having about 5 fewer migraine days each month, compared with baseline. Overall, 62.3% of patients had achieved a reduction of at least 50% in monthly migraine days; 38.4%, a reduction of at least 75%; and 18.5%, a reduction of 100%.
The pattern was similar when it came to monthly migraine-specific medication days, monthly headache days, and monthly migraine attacks, according to Dr. Silberstein.
“The safety and tolerability during the open-label phase were similar to that in the double-blind phase,” he noted. “One of the reasons this is important is that, unlike small molecules, the monoclonal antibodies are not metabolized in the liver, but are metabolized in the reticuloendothelial system. So, the concept of small-molecule toxicity does not exist.”
Amgen sponsored the trial. Dr. Silberstein disclosed that he receives consulting fees from Amgen.
VANCOUVER – An investigational antibody that targets the calcitonin gene-related peptide (CGRP) receptor may be efficacious and safe in the longer term for preventing episodic migraine, data from an ongoing extension of a phase II trial showed.
Among the 483 adults studied, the benefit seen with Amgen’s 334 antibody (AMG 334) at the highest dose at 12 weeks persisted out to a year, according to interim results reported at the annual meeting of the American Academy of Neurology.
At that time, patients on average were having roughly 5 fewer migraine days each month. Nearly two-thirds had experienced at least a halving of monthly migraine days, and nearly one-fifth had become totally migraine free. In addition, the antibody’s good safety profile also held up.
“These data strongly support the further investigation of AMG 334 as a potential preventive medication,” commented presenting author Dr. Stephen Silberstein, professor and director of the headache center at Thomas Jefferson University, Philadelphia.
“If you give a monoclonal antibody to someone with migraine and it takes away the associated features, then you just wind up with a headache day,” Dr. Silberstein noted. “But analysis clearly shows the reduction in migraine days with a concomitant reduction in headache days, showing you are not just removing the associated symptoms.”
It is too early to say whether the antibody is actually modifying the underlying disease, according to Dr. Silberstein. That will require a randomized withdrawal trial, assessing whether patients who stop treatment have a recurrence.
In any event, on the basis of the favorable findings from the phase II trial and its extension, a phase III trial, the Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention (STRIVE), is now underway.
Four antibodies targeting CGRP signaling are now being investigated for migraine prevention, but AMG 334 is the only one targeting the receptor, noted session attendee Dr. Denise E. Chou of the department of neurology at Columbia University and director of the Columbia University headache center, New York.
“Particularly striking is the almost 20% rate of 100% remission. That’s remarkable, because it’s unheard of for any of the current existing migraine therapies,” she noted. The antibody’s longer half-life (translating to greater convenience for patients) and good safety profile are also pluses.
“With the four different antibodies out there, it’s kind of a race,” Dr. Chou commented. “We’ll have to see which have the best efficacy rates and side effect profiles, and which are going to be the best in terms of patient compliance and dosing scheme.”
Compared with some other trials, the AMG 334 trial enrolled a population that had episodic migraine, which is relatively easier to treat, Dr. Chou noted.
“We know, in general, that chronic refractory patients tend to have lower response rates to therapies,” she said. “But it is nice that there are different studies looking at different populations, because that’s what we see, a very heterogeneous clinic population. To be able to reflect the real-world setting is important.”
Patients were eligible for the Amgen-sponsored trial if they had 4-14 migraine days and fewer than 15 headache days per month. They could have experienced failure of up to two previous prophylactic therapies. Rescue medications were allowed, but medication overuse was not.
The patients were randomized 2:2:2:3 to monthly subcutaneous injections of AMG 334 at doses of 7 mg, 21 mg, or 70 mg, or to placebo, on a double-blind basis.
At baseline, the patients had about 9 migraine days and 10 headache days each month so that migraine accounted for most of the total, Dr. Silberstein noted.
At 12 weeks, the change in monthly migraine days – the trial’s primary outcome – was greater with the 70-mg dose than with placebo, with a reduction of 3.40 days vs. 2.28 days each month (P = .021) (Lancet Neurol. 2016 Apr.;15[4]:382-90).
A post hoc analysis suggested that this difference was already significant merely 2 weeks into treatment, Dr. Silberstein reported. The reductions seen with the lower doses were not significant.
All patients were then switched to the 70-mg dose of AMG 334 on the open-label extension, which is planned to continue for up to 5 years. The results showed that both lower-dose groups and the placebo group quickly caught up to their peers on the higher dose when it came to the reduction in monthly migraine days.
“In contrast to both the topiramate and botulinum [toxin] studies, at 16 weeks, there was no difference between patients who had started on the higher dose of [AMG 334] or who were transferred over,” Dr. Silberstein commented. “If one does an individual analysis of patients who were before on placebo or the lower doses, each of them caught up, which suggests a difference in the [mechanism of] … botulinum toxin or topiramate – because with those drugs, they never caught up at the end of 6 months.”
At 52 weeks, the patients as a whole were having about 5 fewer migraine days each month, compared with baseline. Overall, 62.3% of patients had achieved a reduction of at least 50% in monthly migraine days; 38.4%, a reduction of at least 75%; and 18.5%, a reduction of 100%.
The pattern was similar when it came to monthly migraine-specific medication days, monthly headache days, and monthly migraine attacks, according to Dr. Silberstein.
“The safety and tolerability during the open-label phase were similar to that in the double-blind phase,” he noted. “One of the reasons this is important is that, unlike small molecules, the monoclonal antibodies are not metabolized in the liver, but are metabolized in the reticuloendothelial system. So, the concept of small-molecule toxicity does not exist.”
Amgen sponsored the trial. Dr. Silberstein disclosed that he receives consulting fees from Amgen.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: AMG 334 has durable efficacy and safety in reducing episodic migraine.
Major finding: At 1 year, patients were experiencing about 5 fewer migraine days each month on average, and 62.3% had achieved at least a 50% reduction in monthly migraine days.
Data source: An interim analysis of an open-label extension of a phase II trial in 483 adults with episodic migraine.
Disclosures: Amgen sponsored the trial. Dr. Silberstein disclosed that he receives consulting fees from Amgen.
Adjuvant endocrine therapy for premenopausal breast cancer patients should be individualized
Oncologists should take an individualized approach when making decisions about adjuvant endocrine therapies for premenopausal hormone receptor–positive, HER2-negative early breast cancer, suggests an analysis of a pair of randomized phase III trials published online in the Journal of Clinical Oncology.
Investigators led by Meredith M. Regan, Sc.D., of Dana-Farber Cancer Institute in Boston, analyzed data from the TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial) trials of adjuvant endocrine therapies, comprising a total of nearly 5,000 women.
Results suggested that the absolute improvement in the 5-year breast cancer–free interval rate with exemestane plus ovarian function suppression (OFS) versus tamoxifen with or without OFS ranged from less than 1% in women with a lowest recurrence risk based on clinicopathologic factors to 10%-15% in women with a highest risk.
“TEXT and SOFT demonstrated that premenopausal women with hormone receptor–positive disease benefit, on average, from exemestane plus OFS versus tamoxifen with or without OFS. However, individualized treatment decisions should weigh the benefits against the adverse effects and costs of these therapy options,” the investigators wrote.
“In the absence of predictive biomarkers, consideration of a patient’s prognosis, as illustrated by STEPP [Subpopulation Treatment Effect Pattern Plot] analysis of a composite measure of recurrence risk in the TEXT and SOFT populations, is integral to this decision making,” they added.
In the SOFT trial, women were randomized to 5 years of tamoxifen alone (as an active comparator), tamoxifen plus OFS, or exemestane (Aromasin) plus OFS. In the TEXT trial, women were randomized to 5 years of exemestane plus OFS or of tamoxifen plus OFS.
Dr. Regan and colleagues based their analyses on a total of 4,891 women. They assessed each patient’s composite recurrence risk from a Cox model that included a set of conventional clinicopathologic factors: age, nodal status, tumor size and grade, and estrogen receptor, progesterone receptor, and Ki-67 expression levels. And they used STEPP methodology to assess the impact of endocrine therapy across groups having different risk.
The median duration of follow-up was 5.6 years in the SOFT trial and 6 years in the TEXT trial. Results showed that the 5-year breast cancer–free interval rate was 90.8% for the study cohort as a whole. But it ranged considerably from 98.6% for patients with composite risk in the lowest quartile to 77.5% for patients with composite risk in the highest quartiles, the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2015.64.3171).
In the SOFT population, patients who remained premenopausal after neoadjuvant or adjuvant chemotherapy had an absolute improvement of 5% or more in the 5-year breast cancer–free interval rate with exemestane plus OFS, compared with tamoxifen plus OFS or tamoxifen alone. The difference was 10%-15% for the subset at intermediate to high risk for recurrence.
In addition, a benefit of tamoxifen plus OFS over tamoxifen alone was evident in patients having the highest composite risk.
Among patients who were not given chemotherapy, who on average had the lowest composite recurrence risk, the 5-year breast cancer–free interval rate was excellent regardless of the endocrine therapy received.
In the TEXT trial population, the benefit of exemestane plus OFS over tamoxifen plus OFS in 5-year breast cancer–free interval rate ranged from 5% to 15%. Again, the patients who were not given chemotherapy, who had the lowest composite recurrence risk, fared well regardless of which endocrine therapy they received.
These findings should help guide clinical decisions in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, both at the extremes of risk and in the scenario of intermediate risk, where factors such as patient preference, tolerance, and cost play a greater role, according to the investigators.
“Further follow-up of TEXT and SOFT patients is essential to guide patient care,” they concluded.
Oncologists should take an individualized approach when making decisions about adjuvant endocrine therapies for premenopausal hormone receptor–positive, HER2-negative early breast cancer, suggests an analysis of a pair of randomized phase III trials published online in the Journal of Clinical Oncology.
Investigators led by Meredith M. Regan, Sc.D., of Dana-Farber Cancer Institute in Boston, analyzed data from the TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial) trials of adjuvant endocrine therapies, comprising a total of nearly 5,000 women.
Results suggested that the absolute improvement in the 5-year breast cancer–free interval rate with exemestane plus ovarian function suppression (OFS) versus tamoxifen with or without OFS ranged from less than 1% in women with a lowest recurrence risk based on clinicopathologic factors to 10%-15% in women with a highest risk.
“TEXT and SOFT demonstrated that premenopausal women with hormone receptor–positive disease benefit, on average, from exemestane plus OFS versus tamoxifen with or without OFS. However, individualized treatment decisions should weigh the benefits against the adverse effects and costs of these therapy options,” the investigators wrote.
“In the absence of predictive biomarkers, consideration of a patient’s prognosis, as illustrated by STEPP [Subpopulation Treatment Effect Pattern Plot] analysis of a composite measure of recurrence risk in the TEXT and SOFT populations, is integral to this decision making,” they added.
In the SOFT trial, women were randomized to 5 years of tamoxifen alone (as an active comparator), tamoxifen plus OFS, or exemestane (Aromasin) plus OFS. In the TEXT trial, women were randomized to 5 years of exemestane plus OFS or of tamoxifen plus OFS.
Dr. Regan and colleagues based their analyses on a total of 4,891 women. They assessed each patient’s composite recurrence risk from a Cox model that included a set of conventional clinicopathologic factors: age, nodal status, tumor size and grade, and estrogen receptor, progesterone receptor, and Ki-67 expression levels. And they used STEPP methodology to assess the impact of endocrine therapy across groups having different risk.
The median duration of follow-up was 5.6 years in the SOFT trial and 6 years in the TEXT trial. Results showed that the 5-year breast cancer–free interval rate was 90.8% for the study cohort as a whole. But it ranged considerably from 98.6% for patients with composite risk in the lowest quartile to 77.5% for patients with composite risk in the highest quartiles, the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2015.64.3171).
In the SOFT population, patients who remained premenopausal after neoadjuvant or adjuvant chemotherapy had an absolute improvement of 5% or more in the 5-year breast cancer–free interval rate with exemestane plus OFS, compared with tamoxifen plus OFS or tamoxifen alone. The difference was 10%-15% for the subset at intermediate to high risk for recurrence.
In addition, a benefit of tamoxifen plus OFS over tamoxifen alone was evident in patients having the highest composite risk.
Among patients who were not given chemotherapy, who on average had the lowest composite recurrence risk, the 5-year breast cancer–free interval rate was excellent regardless of the endocrine therapy received.
In the TEXT trial population, the benefit of exemestane plus OFS over tamoxifen plus OFS in 5-year breast cancer–free interval rate ranged from 5% to 15%. Again, the patients who were not given chemotherapy, who had the lowest composite recurrence risk, fared well regardless of which endocrine therapy they received.
These findings should help guide clinical decisions in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, both at the extremes of risk and in the scenario of intermediate risk, where factors such as patient preference, tolerance, and cost play a greater role, according to the investigators.
“Further follow-up of TEXT and SOFT patients is essential to guide patient care,” they concluded.
Oncologists should take an individualized approach when making decisions about adjuvant endocrine therapies for premenopausal hormone receptor–positive, HER2-negative early breast cancer, suggests an analysis of a pair of randomized phase III trials published online in the Journal of Clinical Oncology.
Investigators led by Meredith M. Regan, Sc.D., of Dana-Farber Cancer Institute in Boston, analyzed data from the TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial) trials of adjuvant endocrine therapies, comprising a total of nearly 5,000 women.
Results suggested that the absolute improvement in the 5-year breast cancer–free interval rate with exemestane plus ovarian function suppression (OFS) versus tamoxifen with or without OFS ranged from less than 1% in women with a lowest recurrence risk based on clinicopathologic factors to 10%-15% in women with a highest risk.
“TEXT and SOFT demonstrated that premenopausal women with hormone receptor–positive disease benefit, on average, from exemestane plus OFS versus tamoxifen with or without OFS. However, individualized treatment decisions should weigh the benefits against the adverse effects and costs of these therapy options,” the investigators wrote.
“In the absence of predictive biomarkers, consideration of a patient’s prognosis, as illustrated by STEPP [Subpopulation Treatment Effect Pattern Plot] analysis of a composite measure of recurrence risk in the TEXT and SOFT populations, is integral to this decision making,” they added.
In the SOFT trial, women were randomized to 5 years of tamoxifen alone (as an active comparator), tamoxifen plus OFS, or exemestane (Aromasin) plus OFS. In the TEXT trial, women were randomized to 5 years of exemestane plus OFS or of tamoxifen plus OFS.
Dr. Regan and colleagues based their analyses on a total of 4,891 women. They assessed each patient’s composite recurrence risk from a Cox model that included a set of conventional clinicopathologic factors: age, nodal status, tumor size and grade, and estrogen receptor, progesterone receptor, and Ki-67 expression levels. And they used STEPP methodology to assess the impact of endocrine therapy across groups having different risk.
The median duration of follow-up was 5.6 years in the SOFT trial and 6 years in the TEXT trial. Results showed that the 5-year breast cancer–free interval rate was 90.8% for the study cohort as a whole. But it ranged considerably from 98.6% for patients with composite risk in the lowest quartile to 77.5% for patients with composite risk in the highest quartiles, the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2015.64.3171).
In the SOFT population, patients who remained premenopausal after neoadjuvant or adjuvant chemotherapy had an absolute improvement of 5% or more in the 5-year breast cancer–free interval rate with exemestane plus OFS, compared with tamoxifen plus OFS or tamoxifen alone. The difference was 10%-15% for the subset at intermediate to high risk for recurrence.
In addition, a benefit of tamoxifen plus OFS over tamoxifen alone was evident in patients having the highest composite risk.
Among patients who were not given chemotherapy, who on average had the lowest composite recurrence risk, the 5-year breast cancer–free interval rate was excellent regardless of the endocrine therapy received.
In the TEXT trial population, the benefit of exemestane plus OFS over tamoxifen plus OFS in 5-year breast cancer–free interval rate ranged from 5% to 15%. Again, the patients who were not given chemotherapy, who had the lowest composite recurrence risk, fared well regardless of which endocrine therapy they received.
These findings should help guide clinical decisions in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, both at the extremes of risk and in the scenario of intermediate risk, where factors such as patient preference, tolerance, and cost play a greater role, according to the investigators.
“Further follow-up of TEXT and SOFT patients is essential to guide patient care,” they concluded.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Decisions about adjuvant endocrine therapies in premenopausal women with HR–positive, HER2-negative early breast cancer should be individualized.
Major finding: The absolute improvement in 5-year breast cancer–free interval rate with exemestane plus OFS versus tamoxifen with or without OFS ranged from less than 1% for those with lowest recurrence risk to 15% for those with highest recurrence risk.
Data source: An analysis of data from 4,891 women treated in the TEXT and SOFT trials of adjuvant endocrine therapies for premenopausal HR–positive, HER2-negative breast cancer.
Disclosures: Dr. Reagan disclosed that her institution receives research funding from Veridex, OncoGenex, Pfizer, Ipsen, Novartis, Merck, Ferring Pharmaceuticals, Celgene, and AstraZeneca.