User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
Trial data suggest beneficial class effects of SGLT2 inhibitors, including dapagliflozin
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
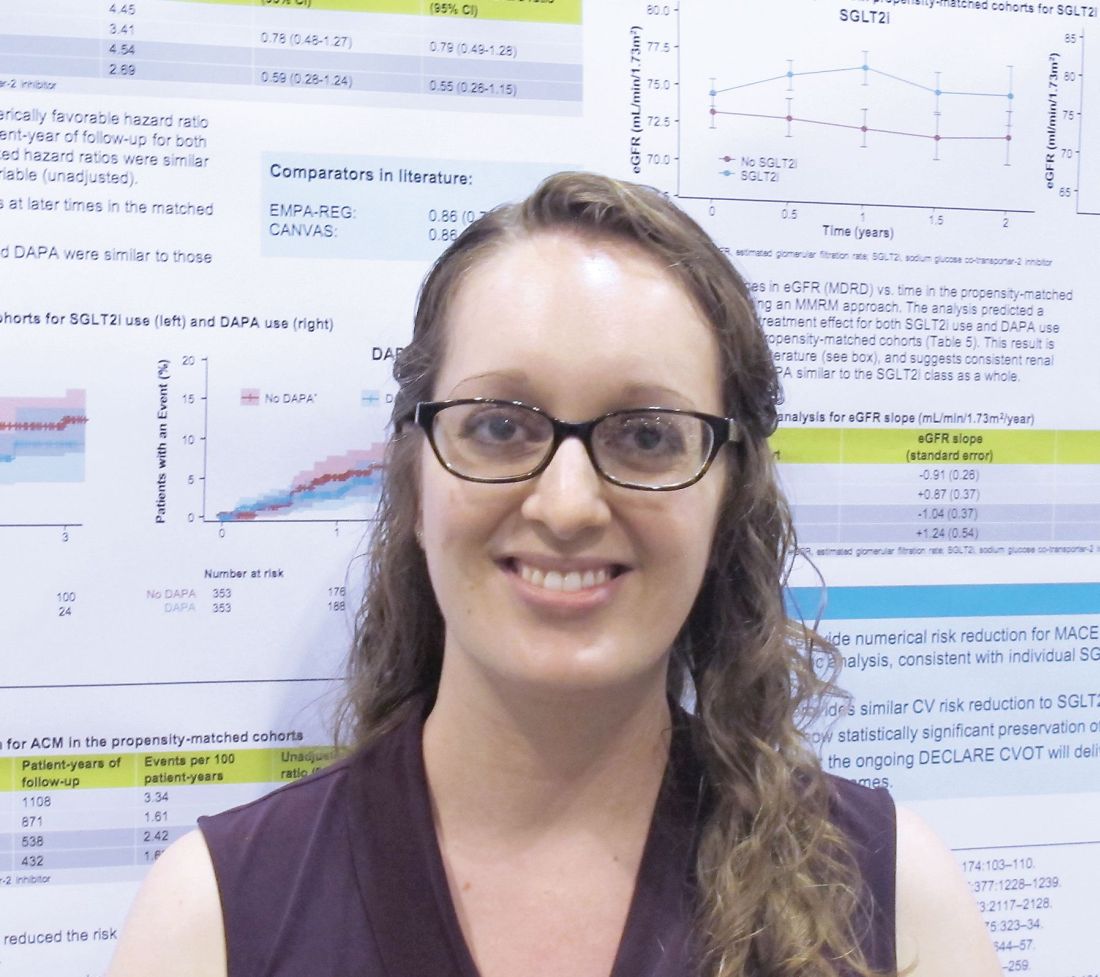
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
REPORTING FROM ADA 2018
Key clinical point: Sodium-glucose transporter 2 inhibitors, including dapagliflozin, have beneficial class effects on major adverse cardiac events, all-cause mortality, and renal function.
Major finding: MACE occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (adjusted hazard ratio, 0.79).
Study details: A post hoc analysis of data from 1,418 EXSCEL trial subjects.
Disclosures: Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
Source: Clegg L et al. ADA 2018, Abstract 130-LB.
CVD-REAL 2: Lower mortality, CV risks with SGLT-2i vs. DPP-4i treatment in T2DM
ORLANDO – according to findings from the CVD-REAL 2 study.
CVD-REAL 2 is a real-world, observational cohort study involving the analysis of health records for two matched cohorts of patients with T2DM from 12 countries across the globe, including 181,620 SGLT-2 inhibitor recipients and 181,620 DPP-4 inhibitor recipients who were newly initiated on their respective treatments between December 2012 and November 2017. The respective rates of all-cause death were 0.83 and 1.33 per 100 patient-years (4,768 events; hazard ratio, 0.51), Shun Kohsaka, MD, of Keio University School of Medicine, Tokyo, and his colleagues reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
“HRs for all-cause death consistently favored SGLT-2 inhibitor vs. DPP-4 inhibitor in each country,” the investigators noted. “Directionally, the same results were observed in other cardiovascular outcomes, including [hospitalization for heart failure (HHF)], and the composite of all-cause death or HHF but modestly for [myocardial infarction] and stroke.”
The rates of hospitalization for heart failure per 100 patient-years were 0.80 and 1.08 in the SGLT-2 inhibitor and DPP-4 inhibitor groups (3,875 events; HR, 0.68), and for HHF plus all-cause death, they were 1.55 and 2.22 per 100 patient-years (7,807 events; HR, 0.67), respectively. The rates of myocardial infarction in the groups, respectively, were 0.53 and 0.58 per 100 patient-years (2,298 events; HR, 0.90), and for stroke, they were 0.82 and 0.99 per 100 patient-years (3,747 events; HR, 0.84), the investigators reported.
Study subjects in both cohorts had a mean age of 58 years, and 30% and 29% in the SGLT-2 inhibitor and DPP-4 inhibitor groups, respectively, had established cardiovascular disease. Only those newly initiated on either an SGLT-2 inhibitor or DPP-4 inhibitor were selected from each data source; fixed-dose combinations were allowed as long as there was no use of either drug during the year prior to enrollment.
In the SGLT-2 inhibitor cohort, most exposures (60.1%) were to dapagliflozin, followed by canagliflozin (23.8%) and empagliflozin (12.1%). The remaining exposures were to ipragliflozin, tofogliflozin, or luseogliflozin (0.3-2.8%). In the DPP-4 inhibitor group, most exposures (49.7%) were to sitagliptin, 18.9% were to linagliptin, 10.4% were to saxagliptin, and the remaining exposures were to alogliptin, gemigliptin, teneligliptin, anagliptin, evogliptin, and trelagliptin (0.1%-4.7%).
Those in the SGLT-2 inhibitor group were followed for a mean of 439 days, and those in the DPP-4 inhibitor group were followed for a mean of 446 days.
“The results were consistent across the subgroups of patients with and without established [cardiovascular disease], favoring SGLT-2 inhibitor vs. DPP-4 inhibitor for all outcomes,” they noted.
Both DPP-4 inhibitors and SGLT2 inhibitors are widely used in T2DM, and although clinical trials demonstrated lower risk of cardiovascular events with SGLT-2 inhibitors and a neutral effect on cardiovascular events with DPP-4 inhibitors, large comparative studies are lacking, the investigators explained.
Though limited by the possibility of residual, unmeasured confounding, as well as by a lack of mortality data in Japan and Singapore apart from the inpatient setting, the findings of this “large, contemporary analysis of real-world administrative data” are complementary to those from previous observational studies and clinical trials, they concluded, noting that “SGLT-2 inhibitor experience in real-world practice is still relatively short and longer-term follow-up is required to examine whether effects are sustained over time.”
The CVD-REAL studies are sponsored by AstraZeneca. Dr. Kohsaka reported receiving research support from Bayer Yakuhin and Daiichi Sankyo and serving on the speaker’s bureau for Bayer Yakuhin and Bristol-Myers Squibb.
SOURCE: Kohsaka S et al. ADA 2018, Abstract 124-LB.
ORLANDO – according to findings from the CVD-REAL 2 study.
CVD-REAL 2 is a real-world, observational cohort study involving the analysis of health records for two matched cohorts of patients with T2DM from 12 countries across the globe, including 181,620 SGLT-2 inhibitor recipients and 181,620 DPP-4 inhibitor recipients who were newly initiated on their respective treatments between December 2012 and November 2017. The respective rates of all-cause death were 0.83 and 1.33 per 100 patient-years (4,768 events; hazard ratio, 0.51), Shun Kohsaka, MD, of Keio University School of Medicine, Tokyo, and his colleagues reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
“HRs for all-cause death consistently favored SGLT-2 inhibitor vs. DPP-4 inhibitor in each country,” the investigators noted. “Directionally, the same results were observed in other cardiovascular outcomes, including [hospitalization for heart failure (HHF)], and the composite of all-cause death or HHF but modestly for [myocardial infarction] and stroke.”
The rates of hospitalization for heart failure per 100 patient-years were 0.80 and 1.08 in the SGLT-2 inhibitor and DPP-4 inhibitor groups (3,875 events; HR, 0.68), and for HHF plus all-cause death, they were 1.55 and 2.22 per 100 patient-years (7,807 events; HR, 0.67), respectively. The rates of myocardial infarction in the groups, respectively, were 0.53 and 0.58 per 100 patient-years (2,298 events; HR, 0.90), and for stroke, they were 0.82 and 0.99 per 100 patient-years (3,747 events; HR, 0.84), the investigators reported.
Study subjects in both cohorts had a mean age of 58 years, and 30% and 29% in the SGLT-2 inhibitor and DPP-4 inhibitor groups, respectively, had established cardiovascular disease. Only those newly initiated on either an SGLT-2 inhibitor or DPP-4 inhibitor were selected from each data source; fixed-dose combinations were allowed as long as there was no use of either drug during the year prior to enrollment.
In the SGLT-2 inhibitor cohort, most exposures (60.1%) were to dapagliflozin, followed by canagliflozin (23.8%) and empagliflozin (12.1%). The remaining exposures were to ipragliflozin, tofogliflozin, or luseogliflozin (0.3-2.8%). In the DPP-4 inhibitor group, most exposures (49.7%) were to sitagliptin, 18.9% were to linagliptin, 10.4% were to saxagliptin, and the remaining exposures were to alogliptin, gemigliptin, teneligliptin, anagliptin, evogliptin, and trelagliptin (0.1%-4.7%).
Those in the SGLT-2 inhibitor group were followed for a mean of 439 days, and those in the DPP-4 inhibitor group were followed for a mean of 446 days.
“The results were consistent across the subgroups of patients with and without established [cardiovascular disease], favoring SGLT-2 inhibitor vs. DPP-4 inhibitor for all outcomes,” they noted.
Both DPP-4 inhibitors and SGLT2 inhibitors are widely used in T2DM, and although clinical trials demonstrated lower risk of cardiovascular events with SGLT-2 inhibitors and a neutral effect on cardiovascular events with DPP-4 inhibitors, large comparative studies are lacking, the investigators explained.
Though limited by the possibility of residual, unmeasured confounding, as well as by a lack of mortality data in Japan and Singapore apart from the inpatient setting, the findings of this “large, contemporary analysis of real-world administrative data” are complementary to those from previous observational studies and clinical trials, they concluded, noting that “SGLT-2 inhibitor experience in real-world practice is still relatively short and longer-term follow-up is required to examine whether effects are sustained over time.”
The CVD-REAL studies are sponsored by AstraZeneca. Dr. Kohsaka reported receiving research support from Bayer Yakuhin and Daiichi Sankyo and serving on the speaker’s bureau for Bayer Yakuhin and Bristol-Myers Squibb.
SOURCE: Kohsaka S et al. ADA 2018, Abstract 124-LB.
ORLANDO – according to findings from the CVD-REAL 2 study.
CVD-REAL 2 is a real-world, observational cohort study involving the analysis of health records for two matched cohorts of patients with T2DM from 12 countries across the globe, including 181,620 SGLT-2 inhibitor recipients and 181,620 DPP-4 inhibitor recipients who were newly initiated on their respective treatments between December 2012 and November 2017. The respective rates of all-cause death were 0.83 and 1.33 per 100 patient-years (4,768 events; hazard ratio, 0.51), Shun Kohsaka, MD, of Keio University School of Medicine, Tokyo, and his colleagues reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
“HRs for all-cause death consistently favored SGLT-2 inhibitor vs. DPP-4 inhibitor in each country,” the investigators noted. “Directionally, the same results were observed in other cardiovascular outcomes, including [hospitalization for heart failure (HHF)], and the composite of all-cause death or HHF but modestly for [myocardial infarction] and stroke.”
The rates of hospitalization for heart failure per 100 patient-years were 0.80 and 1.08 in the SGLT-2 inhibitor and DPP-4 inhibitor groups (3,875 events; HR, 0.68), and for HHF plus all-cause death, they were 1.55 and 2.22 per 100 patient-years (7,807 events; HR, 0.67), respectively. The rates of myocardial infarction in the groups, respectively, were 0.53 and 0.58 per 100 patient-years (2,298 events; HR, 0.90), and for stroke, they were 0.82 and 0.99 per 100 patient-years (3,747 events; HR, 0.84), the investigators reported.
Study subjects in both cohorts had a mean age of 58 years, and 30% and 29% in the SGLT-2 inhibitor and DPP-4 inhibitor groups, respectively, had established cardiovascular disease. Only those newly initiated on either an SGLT-2 inhibitor or DPP-4 inhibitor were selected from each data source; fixed-dose combinations were allowed as long as there was no use of either drug during the year prior to enrollment.
In the SGLT-2 inhibitor cohort, most exposures (60.1%) were to dapagliflozin, followed by canagliflozin (23.8%) and empagliflozin (12.1%). The remaining exposures were to ipragliflozin, tofogliflozin, or luseogliflozin (0.3-2.8%). In the DPP-4 inhibitor group, most exposures (49.7%) were to sitagliptin, 18.9% were to linagliptin, 10.4% were to saxagliptin, and the remaining exposures were to alogliptin, gemigliptin, teneligliptin, anagliptin, evogliptin, and trelagliptin (0.1%-4.7%).
Those in the SGLT-2 inhibitor group were followed for a mean of 439 days, and those in the DPP-4 inhibitor group were followed for a mean of 446 days.
“The results were consistent across the subgroups of patients with and without established [cardiovascular disease], favoring SGLT-2 inhibitor vs. DPP-4 inhibitor for all outcomes,” they noted.
Both DPP-4 inhibitors and SGLT2 inhibitors are widely used in T2DM, and although clinical trials demonstrated lower risk of cardiovascular events with SGLT-2 inhibitors and a neutral effect on cardiovascular events with DPP-4 inhibitors, large comparative studies are lacking, the investigators explained.
Though limited by the possibility of residual, unmeasured confounding, as well as by a lack of mortality data in Japan and Singapore apart from the inpatient setting, the findings of this “large, contemporary analysis of real-world administrative data” are complementary to those from previous observational studies and clinical trials, they concluded, noting that “SGLT-2 inhibitor experience in real-world practice is still relatively short and longer-term follow-up is required to examine whether effects are sustained over time.”
The CVD-REAL studies are sponsored by AstraZeneca. Dr. Kohsaka reported receiving research support from Bayer Yakuhin and Daiichi Sankyo and serving on the speaker’s bureau for Bayer Yakuhin and Bristol-Myers Squibb.
SOURCE: Kohsaka S et al. ADA 2018, Abstract 124-LB.
REPORTING FROM ADA 2018
Key clinical point: SGLT-2 inhibitor treatment is associated with significantly lower risks of death, CV events, and stroke in T2DM, compared with DPP-4 inhibitor treatment.
Major finding: The rates of all-cause death in the SGLT-2 inhibitor and DPP-4 inhibitor groups, respectively, were 0.83 and 1.33 per 100 patient-years (4,768 events; hazard ratio, 0.51).
Study details: A multinational, observational cohort study of more than 360,000 subjects.
Disclosures: The CVD-REAL studies are sponsored by AstraZeneca. Dr. Kohsaka reported receiving research support from Bayer Yakuhin and Daiichi Sankyo and serving on the speaker’s bureau for Bayer Yakuhin and Bristol-Myers Squibb.
Source: Kohsaka S et al. ADA 2018, Abstract 124-LB.
RISE: Insulin glargine, metformin offer no beta cell function benefit in youth
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
EXPERT ANALYSIS AT ADA 2018
CANVAS data: Canagliflozin generally well tolerated up to 6.5 years
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
REPORTING FROM ADA 2018
Key clinical point: Canagliflozin is generally well tolerated for up to 6.5 years in patients with T2DM and high CV risk.
Major finding: Similar differences between canagliflozin and placebo groups for outcomes related to the mechanism of SGLT2 inhibition were seen in the CANVAS and non-CANVAS studies at 6.5 years.
Study details: A comparison of safety outcomes in 10,142 patients in CANVAS and 8,114 patients in non-CANVAS studies.
Disclosures: The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
Source: Hollander P et al. ADA 2018 Abstract 259-OR.
Alteplase, aspirin provide similar functional outcomes after nondisabling stroke
Treatment with alteplase vs. aspirin did not improve functional outcomes at 90 days in patients with minor nondisabling acute ischemic stroke in the randomized phase 3b PRISMS trial.
At 90 days following a minor stroke judged to be nondisabling, a favorable functional outcome – defined as modified Rankin Scale (mRS) score of 0 or 1 – occurred in 122 (78.2%) of 156 patients who received alteplase and in 128 (81.5%) of 157 who received aspirin (adjusted risk difference, –1.1%), wrote Pooja Khatri, MD, of the University of Cincinnati, and her colleagues. The report was published in the July 10 issue of JAMA.
The PRISMS (Potential of rtPA for Ischemic Strokes with Mild Symptoms) trial was intended as a 948-patient, double-blind, placebo-controlled U.S. trial comparing alteplase and aspirin for emergent stroke in patients with National Institutes of Health Stroke Scale (NIHSS) scores of 0-5 at presentation whose stroke-related neurologic deficits were not clearly disabling and in whom the study treatment could be initiated within 3 hours. However, the trial was terminated early by the sponsor – prior to unblinding or interim analyses – because of below-target enrollment. An original plan to measure the difference in favorable functional outcome in the treatment and placebo groups by Cochran-Mantel-Haenszel hypothesis test with stratification by pretreatment NIHSS score, age, and time from onset to treatment was therefore revised to examine the risk difference of the primary outcome by a linear model adjusted for those factors, the authors explained.
Patients in the study had a mean age of 62 years and mean NIHSS score of 2, and were enrolled between May 30, 2014, and Dec. 20, 2016. They received either intravenous alteplase at a standard dose of 0.9 mg/kg with oral placebo, or oral aspirin at a dose of 325 mg with intravenous placebo, and were followed until March 22, 2017.
The primary safety endpoint of the analysis was symptomatic intracranial hemorrhage (sICH) within 36 hours of intravenous alteplase; this occurred in 5 (3.2%) of the alteplase-treated patients and in none of aspirin-treated patients (risk difference, 3.3%).
“Secondary outcomes, including the ordinal analysis of mRS scores (odds ratio, 0.81) and global favorable recovery (OR, 0.86), did not significantly favor either group,” the investigators added.
The findings are noteworthy because more than half of all patients with acute ischemic stroke have minor neurologic deficits, the investigators said, and while prior studies of alteplase included patients with low NIHSS score, few included patients who had no clearly disabling deficits. They added that while “alteplase is the standard of care for patients with ischemic stroke and disabling deficits regardless of severity judged by NIHSS scores, the optimal management of patients with not clearly disabling deficits is unclear.
“The study results raise the hypothesis that even a 6% treatment effect might be unlikely. However, the very early study termination precludes any definitive conclusions,” they wrote, noting that the study has several other limitations, including possible selection bias, relatively high loss to follow-up, and the subjective nature of the definition of “not clearly disabling.”
Additional research may be warranted, they said.
In an editorial, William J. Powers, MD, of the University of North Carolina at Chapel Hill, wrote that despite the limitations of the PRISMS trial, the findings help define the role for intravenous alteplase in the management of acute ischemic stroke.
“Even with early study termination and resultant wide 95% confidence intervals, the excellent outcome in the aspirin group and the numerically similar outcomes between the two groups render it unlikely that intravenous alteplase treatment meaningfully improves functional outcome in patients with initial NIHSS scores of 5 or lower with nondisabling deficits,” he wrote.
He noted, however – as did the study authors – that these conclusions do not apply to all patients with mild stroke. Rather, the findings provide “more certain, but not definitive, evidence” of a lack of benefit with alteplase over aspirin in this patient population (JAMA. 2018;320[2]:141-3).
The findings do suggest that “for these patients, treatment with aspirin along with close monitoring may be an appropriate course of action.”
The PRISMS trial was sponsored by Genentech. Dr. Khatri and many of her colleagues reported receiving personal fees from Genentech, some of which were for serving on the steering committee of the trial. Many investigators reported various financial ties to companies involved in cerebrovascular disease treatment.
Dr. Powers reported having no disclosures.
SOURCE: Khatri P et al. JAMA. 2018;320[2]:156-66.
Treatment with alteplase vs. aspirin did not improve functional outcomes at 90 days in patients with minor nondisabling acute ischemic stroke in the randomized phase 3b PRISMS trial.
At 90 days following a minor stroke judged to be nondisabling, a favorable functional outcome – defined as modified Rankin Scale (mRS) score of 0 or 1 – occurred in 122 (78.2%) of 156 patients who received alteplase and in 128 (81.5%) of 157 who received aspirin (adjusted risk difference, –1.1%), wrote Pooja Khatri, MD, of the University of Cincinnati, and her colleagues. The report was published in the July 10 issue of JAMA.
The PRISMS (Potential of rtPA for Ischemic Strokes with Mild Symptoms) trial was intended as a 948-patient, double-blind, placebo-controlled U.S. trial comparing alteplase and aspirin for emergent stroke in patients with National Institutes of Health Stroke Scale (NIHSS) scores of 0-5 at presentation whose stroke-related neurologic deficits were not clearly disabling and in whom the study treatment could be initiated within 3 hours. However, the trial was terminated early by the sponsor – prior to unblinding or interim analyses – because of below-target enrollment. An original plan to measure the difference in favorable functional outcome in the treatment and placebo groups by Cochran-Mantel-Haenszel hypothesis test with stratification by pretreatment NIHSS score, age, and time from onset to treatment was therefore revised to examine the risk difference of the primary outcome by a linear model adjusted for those factors, the authors explained.
Patients in the study had a mean age of 62 years and mean NIHSS score of 2, and were enrolled between May 30, 2014, and Dec. 20, 2016. They received either intravenous alteplase at a standard dose of 0.9 mg/kg with oral placebo, or oral aspirin at a dose of 325 mg with intravenous placebo, and were followed until March 22, 2017.
The primary safety endpoint of the analysis was symptomatic intracranial hemorrhage (sICH) within 36 hours of intravenous alteplase; this occurred in 5 (3.2%) of the alteplase-treated patients and in none of aspirin-treated patients (risk difference, 3.3%).
“Secondary outcomes, including the ordinal analysis of mRS scores (odds ratio, 0.81) and global favorable recovery (OR, 0.86), did not significantly favor either group,” the investigators added.
The findings are noteworthy because more than half of all patients with acute ischemic stroke have minor neurologic deficits, the investigators said, and while prior studies of alteplase included patients with low NIHSS score, few included patients who had no clearly disabling deficits. They added that while “alteplase is the standard of care for patients with ischemic stroke and disabling deficits regardless of severity judged by NIHSS scores, the optimal management of patients with not clearly disabling deficits is unclear.
“The study results raise the hypothesis that even a 6% treatment effect might be unlikely. However, the very early study termination precludes any definitive conclusions,” they wrote, noting that the study has several other limitations, including possible selection bias, relatively high loss to follow-up, and the subjective nature of the definition of “not clearly disabling.”
Additional research may be warranted, they said.
In an editorial, William J. Powers, MD, of the University of North Carolina at Chapel Hill, wrote that despite the limitations of the PRISMS trial, the findings help define the role for intravenous alteplase in the management of acute ischemic stroke.
“Even with early study termination and resultant wide 95% confidence intervals, the excellent outcome in the aspirin group and the numerically similar outcomes between the two groups render it unlikely that intravenous alteplase treatment meaningfully improves functional outcome in patients with initial NIHSS scores of 5 or lower with nondisabling deficits,” he wrote.
He noted, however – as did the study authors – that these conclusions do not apply to all patients with mild stroke. Rather, the findings provide “more certain, but not definitive, evidence” of a lack of benefit with alteplase over aspirin in this patient population (JAMA. 2018;320[2]:141-3).
The findings do suggest that “for these patients, treatment with aspirin along with close monitoring may be an appropriate course of action.”
The PRISMS trial was sponsored by Genentech. Dr. Khatri and many of her colleagues reported receiving personal fees from Genentech, some of which were for serving on the steering committee of the trial. Many investigators reported various financial ties to companies involved in cerebrovascular disease treatment.
Dr. Powers reported having no disclosures.
SOURCE: Khatri P et al. JAMA. 2018;320[2]:156-66.
Treatment with alteplase vs. aspirin did not improve functional outcomes at 90 days in patients with minor nondisabling acute ischemic stroke in the randomized phase 3b PRISMS trial.
At 90 days following a minor stroke judged to be nondisabling, a favorable functional outcome – defined as modified Rankin Scale (mRS) score of 0 or 1 – occurred in 122 (78.2%) of 156 patients who received alteplase and in 128 (81.5%) of 157 who received aspirin (adjusted risk difference, –1.1%), wrote Pooja Khatri, MD, of the University of Cincinnati, and her colleagues. The report was published in the July 10 issue of JAMA.
The PRISMS (Potential of rtPA for Ischemic Strokes with Mild Symptoms) trial was intended as a 948-patient, double-blind, placebo-controlled U.S. trial comparing alteplase and aspirin for emergent stroke in patients with National Institutes of Health Stroke Scale (NIHSS) scores of 0-5 at presentation whose stroke-related neurologic deficits were not clearly disabling and in whom the study treatment could be initiated within 3 hours. However, the trial was terminated early by the sponsor – prior to unblinding or interim analyses – because of below-target enrollment. An original plan to measure the difference in favorable functional outcome in the treatment and placebo groups by Cochran-Mantel-Haenszel hypothesis test with stratification by pretreatment NIHSS score, age, and time from onset to treatment was therefore revised to examine the risk difference of the primary outcome by a linear model adjusted for those factors, the authors explained.
Patients in the study had a mean age of 62 years and mean NIHSS score of 2, and were enrolled between May 30, 2014, and Dec. 20, 2016. They received either intravenous alteplase at a standard dose of 0.9 mg/kg with oral placebo, or oral aspirin at a dose of 325 mg with intravenous placebo, and were followed until March 22, 2017.
The primary safety endpoint of the analysis was symptomatic intracranial hemorrhage (sICH) within 36 hours of intravenous alteplase; this occurred in 5 (3.2%) of the alteplase-treated patients and in none of aspirin-treated patients (risk difference, 3.3%).
“Secondary outcomes, including the ordinal analysis of mRS scores (odds ratio, 0.81) and global favorable recovery (OR, 0.86), did not significantly favor either group,” the investigators added.
The findings are noteworthy because more than half of all patients with acute ischemic stroke have minor neurologic deficits, the investigators said, and while prior studies of alteplase included patients with low NIHSS score, few included patients who had no clearly disabling deficits. They added that while “alteplase is the standard of care for patients with ischemic stroke and disabling deficits regardless of severity judged by NIHSS scores, the optimal management of patients with not clearly disabling deficits is unclear.
“The study results raise the hypothesis that even a 6% treatment effect might be unlikely. However, the very early study termination precludes any definitive conclusions,” they wrote, noting that the study has several other limitations, including possible selection bias, relatively high loss to follow-up, and the subjective nature of the definition of “not clearly disabling.”
Additional research may be warranted, they said.
In an editorial, William J. Powers, MD, of the University of North Carolina at Chapel Hill, wrote that despite the limitations of the PRISMS trial, the findings help define the role for intravenous alteplase in the management of acute ischemic stroke.
“Even with early study termination and resultant wide 95% confidence intervals, the excellent outcome in the aspirin group and the numerically similar outcomes between the two groups render it unlikely that intravenous alteplase treatment meaningfully improves functional outcome in patients with initial NIHSS scores of 5 or lower with nondisabling deficits,” he wrote.
He noted, however – as did the study authors – that these conclusions do not apply to all patients with mild stroke. Rather, the findings provide “more certain, but not definitive, evidence” of a lack of benefit with alteplase over aspirin in this patient population (JAMA. 2018;320[2]:141-3).
The findings do suggest that “for these patients, treatment with aspirin along with close monitoring may be an appropriate course of action.”
The PRISMS trial was sponsored by Genentech. Dr. Khatri and many of her colleagues reported receiving personal fees from Genentech, some of which were for serving on the steering committee of the trial. Many investigators reported various financial ties to companies involved in cerebrovascular disease treatment.
Dr. Powers reported having no disclosures.
SOURCE: Khatri P et al. JAMA. 2018;320[2]:156-66.
FROM JAMA
Key clinical point: Alteplase does not appear to offer benefit over aspirin in terms of functional outcomes after nondisabling acute ischemic stroke.
Major finding: No significant difference was seen in functional outcomes with alteplase vs. aspirin at 90 days (adjusted risk difference, –1.1%).
Study details: The phase 3b PRISMS trial, involving 313 patients.
Disclosures: Genentech sponsored the trial. Dr. Khatri and many of her colleagues reported receiving personal fees from Genentech, some of which were for serving on the steering committee of the trial. Many investigators reported various financial ties to companies involved in cerebrovascular disease treatment. Dr. Powers reported having no disclosures.
Source: Khatri P et al. JAMA. 2018; 320[2]:156-66.
CANVAS program analysis: Canagliflozin benefits patients with T2DM and poor kidney function
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
REPORTING FROM ADA 2018
Key clinical point: Cardioprotective benefits of canagliflozin in patients with type 2 diabetes mellitus were apparent across varying levels of kidney function.
Major finding: No heterogeneity for treatment effect for the primary major cardiac adverse event endpoint was noted between the high versus low estimated glomerular filtration rate groups (P = .08).
Study details: An analysis of data from the CANVAS program studies involving 10,142 patients.
Disclosures: The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe Pharma.
Source: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
Intensive treatment for T2D pays off in the long run
ORLANDO – Intensified multifactorial treatment proved cost effective over time in type 2 diabetes patients in the practice-changing Steno-2 study, according to 21-year follow-up data from the randomized Danish study.
Cumulative direct health care costs from the start of the trial in 1993 through 2014 were about $13 million in 24 patients in the intensive treatment group who were available for follow-up, and about $12.3 million in 42 patients in the conventional treatment group. The difference in costs between the groups was not statistically significant, Joachim Gaede reported at the annual scientific sessions of the American Diabetes Association.
Costs per patient-year during 1996-2014, however, were significantly lower in the intensive treatment group ($9,648 vs. $10, 681, respectively), said Mr. Gaede, a graduate student in the medicine program at the University of Copenhagen.
Furthermore, patients in the intensified treatment group lived a median of 7.9 years longer than did those who were in the conventional treatment group, suggesting that while costs might be higher early on, investing in early intensified treatment of all known modifiable risk factors in high-risk patients will prolong life and still save money over time thanks to reduced complication-related costs, he noted.
Steno-2 was an open, parallel group study initiated in 1993 to compare conventional multifactorial treatment of type 2 diabetes mellitus (T2DM) with an intensified approach over an 8-year period. Enrollment included 160 patients with high-risk type 2 diabetes. After the primary composite cardiovascular endpoint was assessed, the trial continued as an observational study, with all patients given the intensified, multifactorial treatment that consisted of lifestyle measures and medications targeting hyperglycemia, hypertension, hypercholesterolemia, and hypercoagulation.
Reports from the study over the years led to changes in treatment guidelines to promote more intensive multifactorial treatment, Mr. Gaede said. For example, the initial results reported in 1999 showed a 50% relative risk reduction in kidney, eye, and nerve complications after 4 years with intensive versus conventional treatment; a 2003 report showed a 53% relative risk reduction in MI, stroke, and amputation after 8 years; and a 2008 report demonstrated a 46% relative risk reduction in death after 13 years. Finally, in 2016 a 7.9-year gain in lifespan after 21 years with intensive versus conventional treatment was reported.
In this video interview, Mr. Gaede, junior lead study author, discusses the Steno-2 study findings and the current cost analysis data.
“The bottom line is that ... you can actually, as a patient, be treated at a specialized diabetes clinic ... and, in the long run, it doesn’t cost you anything” more, he said, explaining that the up-front costs of intensive treatment are offset by the money saved because of the reduced complications over time.
Mr. Gaede reported having no disclosures.
SOURCE: Gaede J et al. ADA 2018, Abstract 162-OR.
ORLANDO – Intensified multifactorial treatment proved cost effective over time in type 2 diabetes patients in the practice-changing Steno-2 study, according to 21-year follow-up data from the randomized Danish study.
Cumulative direct health care costs from the start of the trial in 1993 through 2014 were about $13 million in 24 patients in the intensive treatment group who were available for follow-up, and about $12.3 million in 42 patients in the conventional treatment group. The difference in costs between the groups was not statistically significant, Joachim Gaede reported at the annual scientific sessions of the American Diabetes Association.
Costs per patient-year during 1996-2014, however, were significantly lower in the intensive treatment group ($9,648 vs. $10, 681, respectively), said Mr. Gaede, a graduate student in the medicine program at the University of Copenhagen.
Furthermore, patients in the intensified treatment group lived a median of 7.9 years longer than did those who were in the conventional treatment group, suggesting that while costs might be higher early on, investing in early intensified treatment of all known modifiable risk factors in high-risk patients will prolong life and still save money over time thanks to reduced complication-related costs, he noted.
Steno-2 was an open, parallel group study initiated in 1993 to compare conventional multifactorial treatment of type 2 diabetes mellitus (T2DM) with an intensified approach over an 8-year period. Enrollment included 160 patients with high-risk type 2 diabetes. After the primary composite cardiovascular endpoint was assessed, the trial continued as an observational study, with all patients given the intensified, multifactorial treatment that consisted of lifestyle measures and medications targeting hyperglycemia, hypertension, hypercholesterolemia, and hypercoagulation.
Reports from the study over the years led to changes in treatment guidelines to promote more intensive multifactorial treatment, Mr. Gaede said. For example, the initial results reported in 1999 showed a 50% relative risk reduction in kidney, eye, and nerve complications after 4 years with intensive versus conventional treatment; a 2003 report showed a 53% relative risk reduction in MI, stroke, and amputation after 8 years; and a 2008 report demonstrated a 46% relative risk reduction in death after 13 years. Finally, in 2016 a 7.9-year gain in lifespan after 21 years with intensive versus conventional treatment was reported.
In this video interview, Mr. Gaede, junior lead study author, discusses the Steno-2 study findings and the current cost analysis data.
“The bottom line is that ... you can actually, as a patient, be treated at a specialized diabetes clinic ... and, in the long run, it doesn’t cost you anything” more, he said, explaining that the up-front costs of intensive treatment are offset by the money saved because of the reduced complications over time.
Mr. Gaede reported having no disclosures.
SOURCE: Gaede J et al. ADA 2018, Abstract 162-OR.
ORLANDO – Intensified multifactorial treatment proved cost effective over time in type 2 diabetes patients in the practice-changing Steno-2 study, according to 21-year follow-up data from the randomized Danish study.
Cumulative direct health care costs from the start of the trial in 1993 through 2014 were about $13 million in 24 patients in the intensive treatment group who were available for follow-up, and about $12.3 million in 42 patients in the conventional treatment group. The difference in costs between the groups was not statistically significant, Joachim Gaede reported at the annual scientific sessions of the American Diabetes Association.
Costs per patient-year during 1996-2014, however, were significantly lower in the intensive treatment group ($9,648 vs. $10, 681, respectively), said Mr. Gaede, a graduate student in the medicine program at the University of Copenhagen.
Furthermore, patients in the intensified treatment group lived a median of 7.9 years longer than did those who were in the conventional treatment group, suggesting that while costs might be higher early on, investing in early intensified treatment of all known modifiable risk factors in high-risk patients will prolong life and still save money over time thanks to reduced complication-related costs, he noted.
Steno-2 was an open, parallel group study initiated in 1993 to compare conventional multifactorial treatment of type 2 diabetes mellitus (T2DM) with an intensified approach over an 8-year period. Enrollment included 160 patients with high-risk type 2 diabetes. After the primary composite cardiovascular endpoint was assessed, the trial continued as an observational study, with all patients given the intensified, multifactorial treatment that consisted of lifestyle measures and medications targeting hyperglycemia, hypertension, hypercholesterolemia, and hypercoagulation.
Reports from the study over the years led to changes in treatment guidelines to promote more intensive multifactorial treatment, Mr. Gaede said. For example, the initial results reported in 1999 showed a 50% relative risk reduction in kidney, eye, and nerve complications after 4 years with intensive versus conventional treatment; a 2003 report showed a 53% relative risk reduction in MI, stroke, and amputation after 8 years; and a 2008 report demonstrated a 46% relative risk reduction in death after 13 years. Finally, in 2016 a 7.9-year gain in lifespan after 21 years with intensive versus conventional treatment was reported.
In this video interview, Mr. Gaede, junior lead study author, discusses the Steno-2 study findings and the current cost analysis data.
“The bottom line is that ... you can actually, as a patient, be treated at a specialized diabetes clinic ... and, in the long run, it doesn’t cost you anything” more, he said, explaining that the up-front costs of intensive treatment are offset by the money saved because of the reduced complications over time.
Mr. Gaede reported having no disclosures.
SOURCE: Gaede J et al. ADA 2018, Abstract 162-OR.
REPORTING FROM ADA 2018
KEYNOTE-427: Pembrolizumab monotherapy shows promise in accRCC
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
REPORTING FROM ASCO 2018
Key clinical point: Pembrolizumab monotherapy shows promising efficacy and tolerability in accRCC.
Major finding: Overall response rate was 38%.
Study details: The phase 2 KEYNOTE-427 trial of 110 patients from one of two study cohorts.
Disclosures: Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
Source: McDermott DF et al. ASCO 2018, Abstract 4500.
Eversense CGM shown safe, accurate for 180 days in adolescents
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
REPORTING FROM ADA 2018
Key clinical point: The Eversense fully implantable continuous glucose monitoring device is safe and accurate in adolescents.
Major finding: The MARD between sensor and true laboratory glucose values showed high device accuracy, at 9.4% over 180 days.
Study details: A prospective single-arm study of 30 children and 6 adults.
Disclosures: Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
Source: Aronson R et al. ADA Abstract 13-OR.
Average glucose, A1c discordance is common, highlights ADAG equation concerns
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
REPORTING FROM ADA 2018
Key clinical point: AG and A1c discordance is common, thus professional consensus regarding acceptable discordance is needed before ADAG equation use is broadened.
Major finding: CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04).
Study details: A retrospective review of 1,039 patient charts.
Disclosures: Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
Source: Perlman J et al. ADA 2018 Abstract 12-OR.