User login
How common is IUD perforation, expulsion, and malposition?
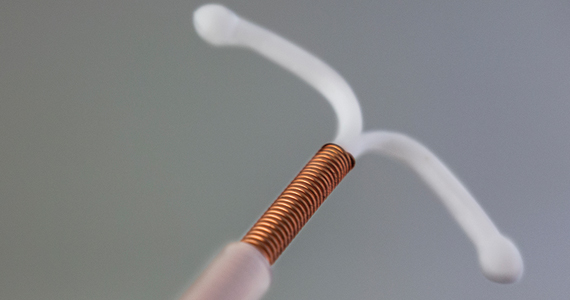
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
Will NAAT replace microscopy for the identification of organisms causing vaginitis?
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
Over the past 200 years, identification of the specific organism causing an infection has evolved from a reliance on patient history and physical examination to the use of microscopic examination of relevant biological samples to the rise of microbial culture and immunological testing as the gold standards for diagnosis. More recently, advances in nucleic acid testing have made nucleic acid amplification testing (NAAT) a primary method for identifying the specific organism causing an infection.
The evolution of the diagnosis of gonorrhea in clinical practice is a good example of the inexorable evolution of diagnostic techniques from physical examination to microscopic analysis to culture and finally to NAAT. Neiseer discovered Neisseria gonorrhea in 1879.1 In 19th century general medical practice gonorrhea was often diagnosed based on history and physical examination and sometimes microscopy was also utilized.2 In the mid-20th century, it was realized that culture was a superior approach to diagnosis of gonorrhea, and it became the gold standard for diagnosis in general practice.3 NAAT has now replaced culture as the gold standard for the diagnosis of gonorrhea because of its superior performance in clinical practice.4 It may now be time to consider using NAAT rather than microscopy and culture in general practice for the identification of specific microorganisms causing vaginitis.
Trichomoniasis
Vaginitis caused by Trichomonas vaginalis is characterized by a discharge that is foamy and green-yellow in color, with a vaginal pH that is >4.5. Microscopy of a vaginal specimen has low sensitivity, in the range of 50%, for detecting T vaginalis.5-7 There are many factors that make microscopy a poor approach to the diagnosis of T vaginalis, including the rapid decrease in protozoan motility once a vaginal specimen is placed on a glass slide and the similar size of non-motile T vaginalis and other cells in the vagina.
Given the low sensitivity of microscopy for the diagnosis of trichomoniasis, the American College of Obstetricians and Gynecologists (ACOG) recommends NAAT as a primary approach to test for T vaginalis, with culture or NAAT testing as alternative approaches.8 The Centers for Disease Control and Prevention (CDC) recommends that if a wet mount is negative for T vaginalis that NAAT should be utilized.9
In this 2-step testing process, the first step is to test the vaginal pH and perform a microscopic examination of a vaginal specimen for T vaginalis. If T vaginalis organisms are detected, the diagnosis of trichomoniasis is confirmed. If organisms are not detected the second step would be to send a vaginal or urine specimen for NAAT for T vaginalis or for culture. An advantage of NAAT over culture is that urine specimens can be used for diagnosis of T vaginalis while urine specimens are not suitable for culture because of low sensitivity. For patients diagnosed with trichomoniasis, the CDC recommends that testing be repeated in 3 months because of high rates of reinfection. NAAT would be an optimal test to use in this situation.
Continue to: Bacterial vaginosis and candidiasis...
Bacterial vaginosis and candidiasis
ACOG recommends using Amsel criteria or Nugent scoring of a specimen colorized with a Gram stain for the diagnosis of bacterial vaginosis and microscopy or culture for the diagnosis of candidiasis.8 Recent research reports that NAAT testing for bacterial vaginosis and candidiasis may be more sensitive than standard office-based approaches for detecting these two causes of vaginitis. In a study of approximately 1,740 patients with symptoms of vaginitis, vaginal specimens were analyzed using NAAT or standard office approaches to diagnosis.10 In this study the diagnostic gold standards were Nugent scoring with Amsel criteria to resolve intermediate Nugent scores for bacterial vaginosis and culture for Candida. The study demonstrated the superiority of NAAT testing over standard office approaches for the identification of the cause of the vaginitis. NAAT testing was reported to have superior sensitivity for diagnosing bacterial vaginosis compared with the original Amsel criteria (93% vs 76%, respectively (P <.0001), with similar respective specificities of 92% and 94% .10 NAAT testing also had superior sensitivity for diagnosing Candidiasis compared with microscopy after potassium hydroxide treatment of a vaginal specimen (91% vs 58%, respectively (P <.0001).10 NAAT testing also had superior specificity compared with microscopy after potassium hydroxide treatment of a vaginal specimen (94% vs 89%, respectively (P < .0005).10
In another study comparing NAAT with clinical diagnosis for 466 patients with symptoms of vaginitis, standard office approaches to the diagnosis of vaginitis resulted in the failure to identify the correct infection in a large number of cases. For the diagnosis of bacterial vaginosis, clinicians missed 42% of the cases identified by NAAT. For the diagnosis of Candida, clinicians missed 46% of the cases identified by NAAT. For T vaginalis diagnosis, clinicians missed 72% of the cases identified by NAAT. Clearly, this resulted in clinicians not treating many infections detected by NAAT.11
Continue to: One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis...
One in 5 patients with symptoms of vaginitis have 2 causes of vaginitis
In a recent study, 1,471 patients with a symptom of vaginitis (abnormal vaginal discharge, itching or irritation, or odor) self-collected a vaginal swab and had a vaginal swab collected by a clinician.12 The swabs were placed in buffer and the samples were tested by NAAT using the BD Max system (Franklin Lakes, New Jersey) for the presence of nucleic acid sequences of the microorganisms responsible for the most common causes of vaginitis. In this cohort, using the clinician collected vaginal swabs for NAAT, the investigators reported the following pattern of detection of nucleic acid sequences: 36.1%, bacterial vaginosis pattern; 16.2%, Candida spp.; 1.6%, T vaginalis; 0.7%, Candida glabrata; and 0.1%, Candida krusei. Nucleic acid sequences of multiple organisms were detected in 21.7% of patients, including 13.9% with bacterial vaginosis pattern plus Candida spp., 4.9% with bacterial vaginosis pattern plus T vaginalis, 0.3% with Candida spp. plus T vaginalis, 0.2% with Candida spp. plus Candida glabrata, 0.2% with bacterial vaginosis pattern plus Candida glabrata, and 2.2% with all 3 organisms. A total of 23.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.
In another study of 1,491 patients with a symptom of vaginitis, clinician-collected vaginal swabs were tested by NAAT using the Aptima BV and Aptima Candida/Trichomonas systems (Hologic, Marlborough, Massachusetts) for the presence of nucleic acid sequences of microorganisms responsible for most cases of vaginitis.13 The investigators reported the following pattern of detection of nucleic acid sequences: 28.6%, bacterial vaginosis pattern; 14.2%, Candida spp.; 3%, T vaginalis; 1.9%, Candida glabrata.13 Nucleic acid sequences from multiple organisms were detected in 23.3% of patients. Nucleic acid sequences suggesting the presence of two different causes of vaginitis were detected among 20.8% of patients, including bacterial vaginosis plus Candida spp., 11.1%; bacterial vaginosis plus T vaginalis, 7.2%; Candida spp. plus T vaginalis, 1.0%; Candida spp. plus Candida glabrata, 0.9%; bacterial vaginosis plus Candida spp., 0.5%; Candida glabrata plus T vaginalis, 0.1%. Nucleic acid sequences suggesting the presence of 3 different causes of vaginitis were detected in 2.4% of patients, the most common being the combination of bacterial vaginosis plus Candida spp. plus T vaginalis, 1.7% and bacterial vaginosis plus Candida spp. plus Candida glabrata, 0.5%. Nucleic acid sequences suggesting the presence of 4 different causes of vaginitis were detected in 0.1% of patients. A total of 28.8% of the women had no detectable nucleic acid sequences associated with organisms known to cause vaginitis.13
In clinical practice it is uncommon to see the diagnosis of multiple causes of vaginitis recorded in the medical record of a patient. This suggests that we are not effectively identifying the 20% of patients with multiple causes of vaginitis.
When multiple organisms that cause vaginitis are present, NAAT is superior to clinical evaluation for diagnosis
In a study of 1,264 patients with symptoms of vaginitis who had an identified microbial cause, more than 20% had multiple organisms detected by NAAT
Patient collection of a vaginal swab for NAAT
Multiple studies have reported that collection of a vaginal swab for NAAT by the patient or a clinician results in similar excellent test performance.4,12,13 This observation might catalyze the development of clinical protocols where patients with vaginitis could collect the swab for NAAT analysis, without needing to have a speculum examination by a clinician.
When collecting a vaginal specimen for NAAT it is important that no vaginal lubricants or creams contaminate the collection swab. Vaginal lubricants and creams may inhibit the polymerase chain reaction enzymes resulting in a false negative. The swab may be directly inserted into the vagina to collect the specimen or a speculum without a lubricant, except water can be used to facilitate specimen collection. To collect a specimen without a speculum the swab is inserted 2 inches into the vagina and rotated for 10 to 15 seconds.
What should clinicians do while waiting for a NAAT result?
A major problem with NAAT testing for vaginitis is that the results are not available at the initial patient visit, impacting the ability to make an immediate diagnosis and provide targeted antibiotic treatment. Given that bacterial vaginosis and Candida species are the most common causes of infectious vaginitis in many populations of gynecology patients, one approach is to initiate treatment with one dose of an oral antifungal agent and a multiday course of vaginal metronidazole. Once the NAAT test results are available, the treatment can be refined to specific infectious agents identified by the test, or the antibiotics can be discontinued if no relevant microorganisms are detected. Another approach would be to wait until the NAAT test is completed and then prescribe the appropriate antibiotic. My sense is that most patients would not favor this wait and see approach.
Barriers to the use of NAAT for vaginitis
A barrier to the use of NAAT for the diagnosis of vaginitis is that leading organizations do not currently recommend NAAT as a primary approach to diagnosis, favoring microscopy and measurement of vaginal pH.9 In addition, clinicians and patients may be rightfully concerned about the cost of NAAT, which can be substantial.
Vaginitis, especially when it is recurrent, can be stressful14 and have an impact on a patient’s quality of life15,16 and sexual health.17 Arguably, our current practice algorithms for diagnosing the cause of vaginitis are not optimized.18 Our failure to accurately diagnose the cause of vaginitis contributes to inappropriate antibiotic treatment and return visits because of inadequate initial treatment.18 We can improve and simplify our approach to the diagnosis of vaginitis by prioritizing the use of NAAT.19 In turn, reliably making the right diagnosis will result in the optimization of treatment. ●
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
- Jose PP, Vivekanandan V, Sobhanakumari K. Gonorrhea: Historical outlook. J Skin Sex Transm Dis. 2020;2:110-114.
- Bayly HW. The diagnosis and treatment of chronic gonorrhoea and its local complications. Br Med J. 1914;14:584-587.
- Stuart RD. The diagnosis and control of gonorrhoea by bacteriological cultures: with a preliminary report on a new method for transporting clinical material. Glasgow Med J. 1946;27:131-142.
- Wilson JD, Wallace HE, Loftus-Keeling M, et al. Swab-yourself trial with economic monitoring and testing for infections collectively (SYSTEMATIC): Part 2. A diagnostic accuracy and cost-effectiveness study comparing rectal, pharyngeal and urogenital samples analyzed individually, versus as a pooled specimen, for the diagnosis of gonorrhea and chlamydia. Clin Infect Dis. 2021;73:e3183-3193.
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new NAAT: association with ethnicity, symptoms and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312-316.
- Roth AM, Williams JA, Ly R. et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398-400.
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infection. 2013;89:434-438.
- Vaginitis in nonpregnant patients. ACOG Practice Bulletin No 215. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e1-e17.
- Workowksi KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines 2021. MMWR. 2021;70:1-187.
- Schwebke JR, Gaydos CA, Hyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of vaginal panel assay compared with clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130:181-189.
- Schwebke JR, Taylor SN, Ackerman N, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginalis assays: results from a prospective multi-center study. J Clin Microbiol. 2020;58:e01643-19.
- Ehrstrom S, Kornfeld D, Rylander E. Perceived stress in women with recurrent vulvovaginal candidiasis. J Psychosomatic Obstet Gynecol. 2007;28:169-176.
- Abellea S, Guelfucci F, Wagner J, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis in Europe and the USA. Health Quality Life Outcomes. 2013;11:169.
- Fukazawa EI, Witkin SS, Robial R, et al. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch Gynecol Obstet. 2019;300:647-650.
- Giraldo PC, Polpeta NC, Juliato CT, et al. Evaluation of sexual function in Brazilian women with recurrent vulvovaginal candidiasis and localized provoked vulvodynia. J Sex Med. 2012;9:805-811.
- Hillier SL, Austin M, Macio I, et al. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72:1538-1543.
- . Sobel JD. Syndromic treatment of women with vulvovaginal symptoms in the United States: a call to action. Clin Infect Dis. 2021;72:1544-1545.
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.
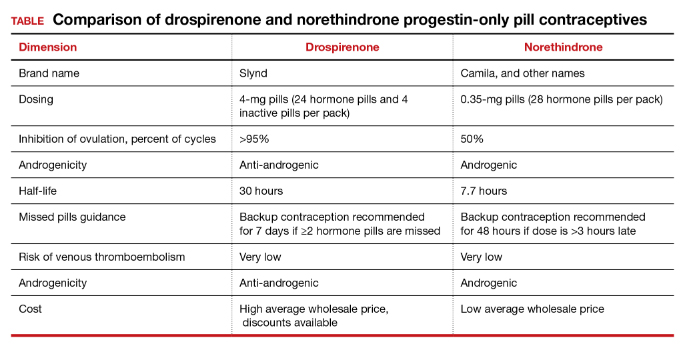
Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Individualize the duration of postpartum magnesium treatment for patients with preeclampsia to best balance the benefits and harms of treatment
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15
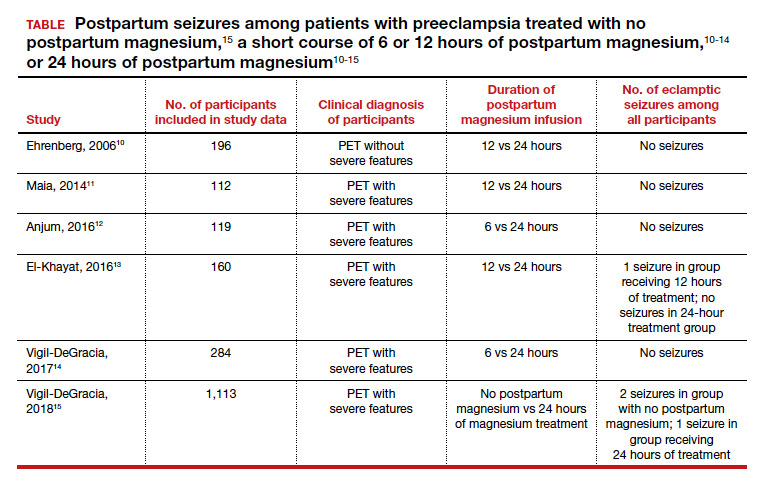
Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Reduce the use of perioperative opioids with a multimodal pain management strategy
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.
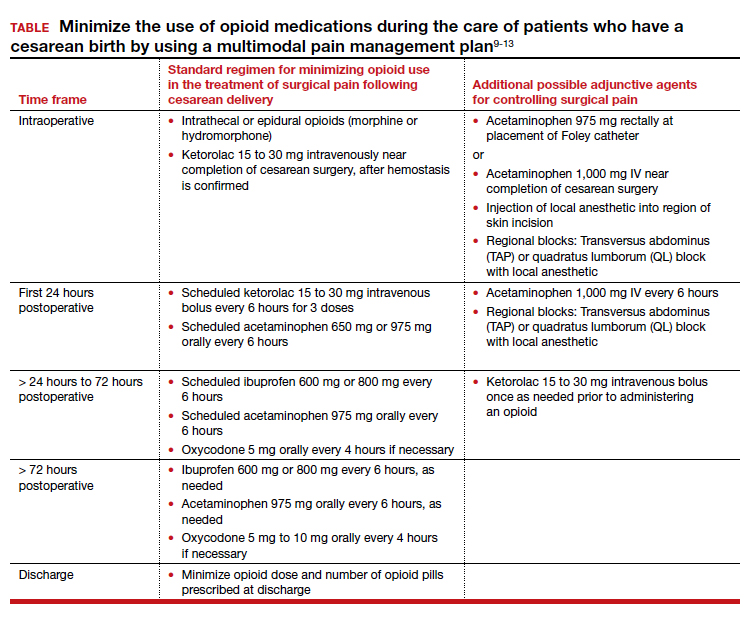
Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
How to screen for prediabetes and type 2 diabetes in an ObGyn practice
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5
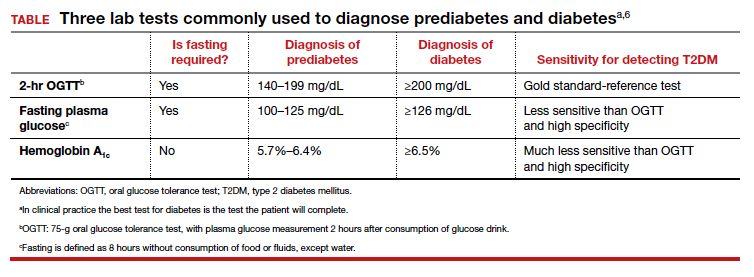
Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
Can we return to the ABCs of crafting a medical record note?
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
Relugolix combination therapy: A novel hormonal treatment for AUB associated with uterine fibroids
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.
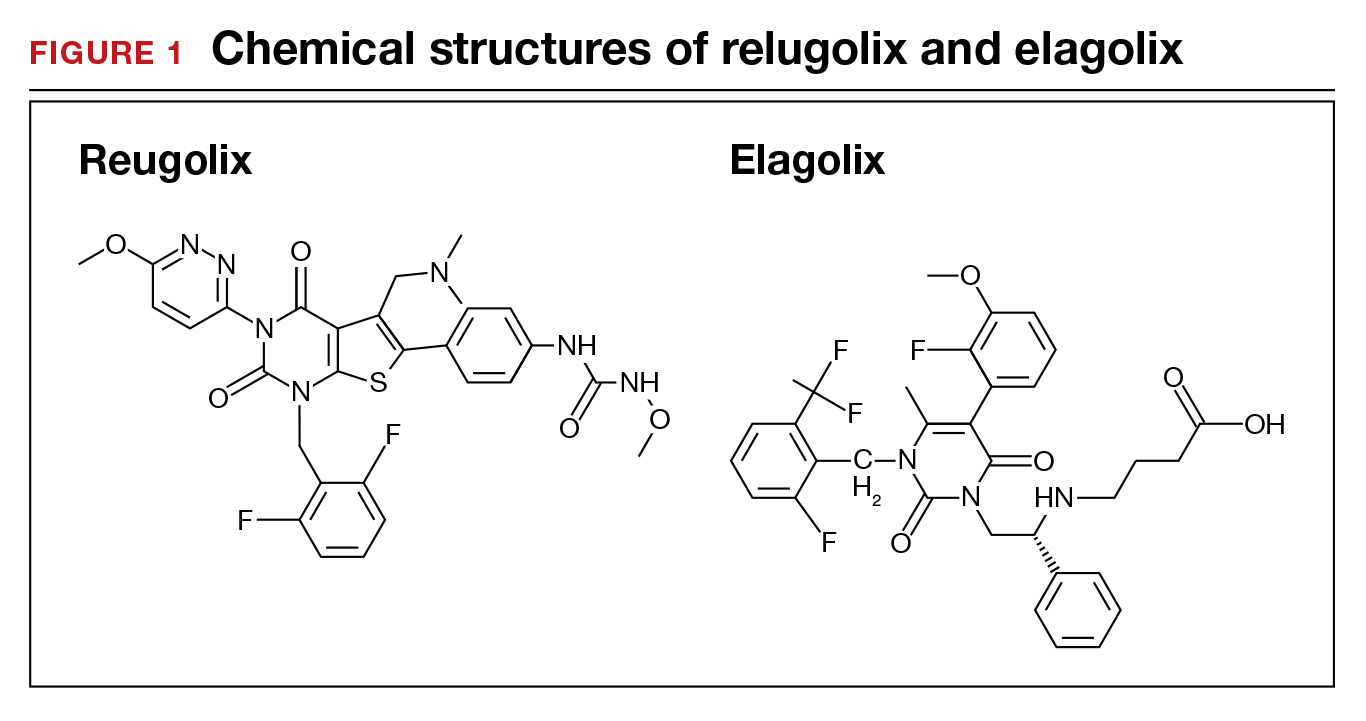
Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.
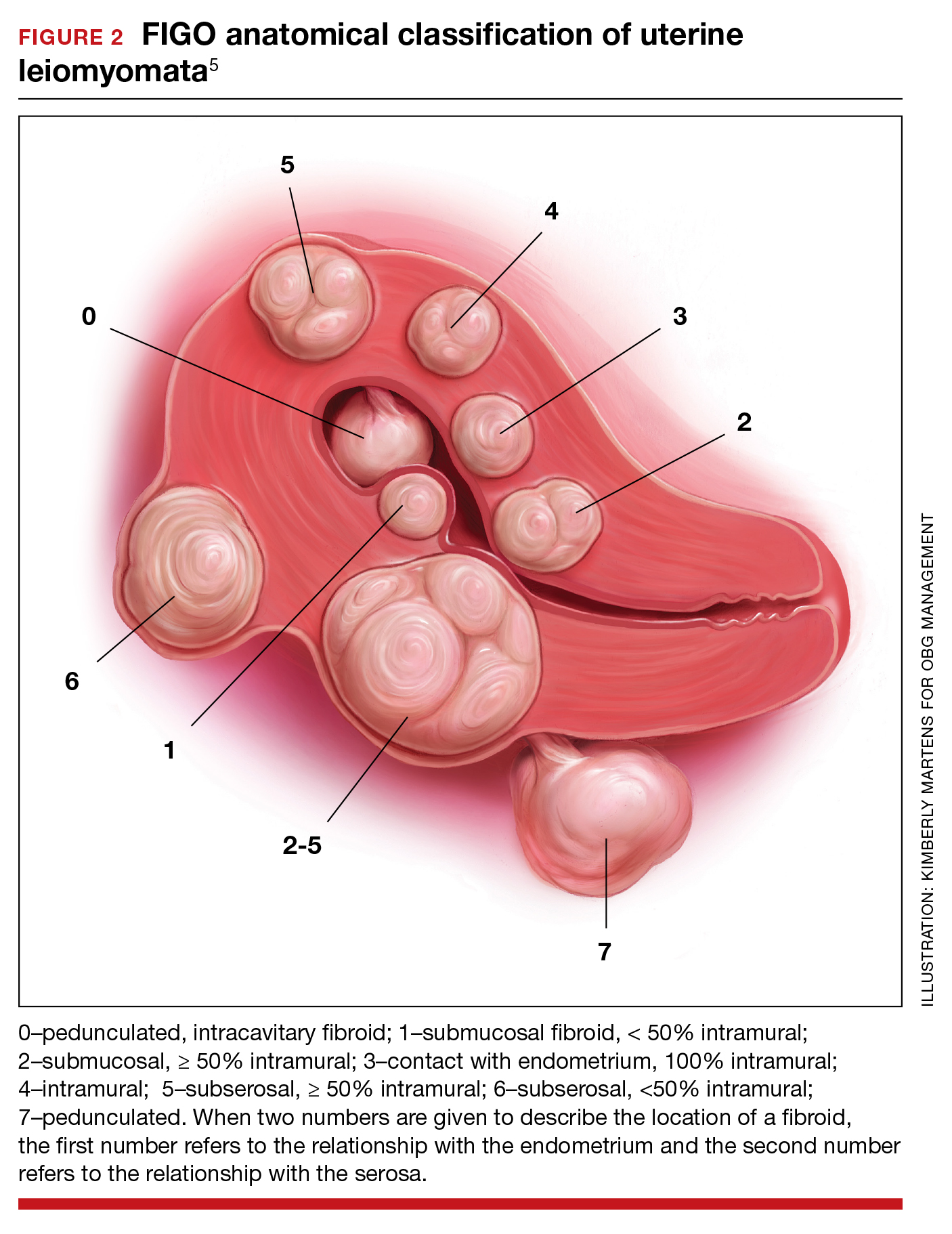
The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
Colorectal cancer screening, 2021: An update
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.
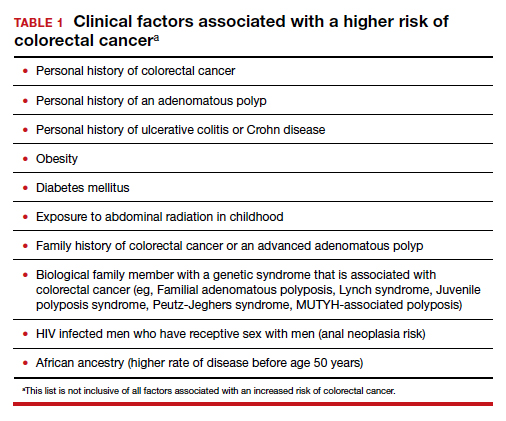
Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).
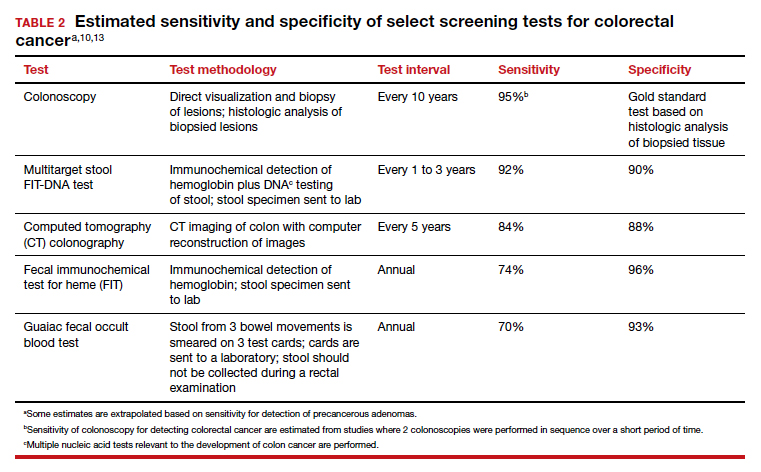
In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.
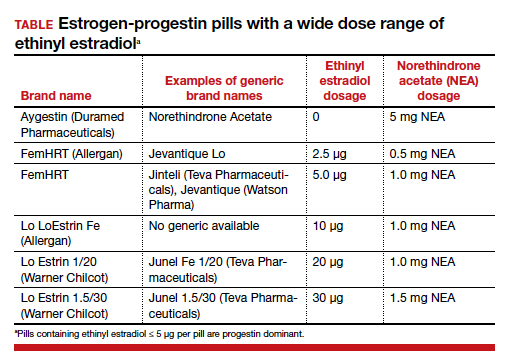
CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.