User login
NOTES Trial Assesses Cholecystectomy Approaches
In the ongoing effort to limit the physical impact of surgery on the human body, researchers are taking minimally invasive gallbladder removal to the next logical step. Instead of removing the organ through the abdominal wall, surgeons in the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR) are performing cholecystectomy through the mouth or the vagina.
Earlier this year, transoral and transvaginal cholecystectomies were performed by Dr. Santiago Horgan and Dr. John Romanelli, respectively, as part of a new clinical trial designed to provide the first multicenter, prospective, randomized, controlled, noninferiority evaluation of these two natural orifice methods with the laparoscopic approach.
Cholecystectomy, usually for benign gallstone disease, is one of the most common surgical procedures; roughly 750,000 such operations are performed annually in the United States. The goal of natural orifice surgery is to eliminate the need to pierce the abdominal wall during gallbladder removal, thereby lessening the risk of complications and additional pain.
About the Trial
The trial (NCT01171027) was announced in July 2010 and began this year. It has an estimated enrollment of approximately 150 patients. The primary outcome measure is to assess the safety and efficacy of transgastric and transvaginal cholecystectomy, compared with conventional laparoscopic cholecystectomy up to 4 weeks after surgery.
The secondary outcome is to assess pain associated with the oral and the vaginal techniques, as compared with conventional laparoscopic cholecystectomy up to 4 weeks post surgery. Cosmesis associated with transgastric and transvaginal cholecystectomy will also be compared with conventional laparoscopic cholecystectomy as well as costs.
The ultimate goal is to develop the technology further so that the transoral and transgastric approaches would not require any incisions in the abdominal wall. Patient recruitment information is available at the NOSCAR website.
When asked to comment on the trial, Dr. David W. Rattner, chief of the division of gastrointestinal and general surgery, Massachusetts General Hospital, Boston, stated: "It is important to note that while over 5,000 transvaginal cholecystectomies have been performed worldwide, this is the first prospective, randomized study to compare NOTES [natural orifice translumenal endoscopic surgery] approaches to a standard therapy for benign gallstone disease. Principal investigators Dr. Michael Kochman at the University of Pennsylvania, Philadelphia, and Dr. Steven L. Schwaitzberg at Harvard University, Cambridge, initiated all of the hard work to make this important comparative trial happen."
Surgical Approaches
The transgastric cholecystectomy is being performed through the use of an orally introduced flexible endoscope to provide working channels and visualization, followed by a gastrotomy made through the stomach wall, allowing the endoscope to pass into the abdominal cavity. The gallbladder is removed from the abdominal cavity into the stomach and ultimately out of the mouth.
The first such operation in the study was performed by Dr. Horgan of the University of California, San Diego. The transvaginal approach is being performed using a posterior colpotomy and a flexible endoscope instead of a conventional laparoscope. The gallbladder is removed from the vaginal incision. The first such operation in the study was performed by Dr. Romanelli of the Baystate Medical Center in Springfield, Mass.
Both approaches are being compared with a version of traditional laparoscopic cholecystectomy in which a laparoscope is introduced through a 1-cm incision in or around the umbilicus, usually with three additional 0.5- to 1.0-cm incisions employed for surgical instrumentation. The gallbladder is typically removed through the umbilical port. According to the clinical trial summary, "the most devastating complication is injury to the major bile ducts." The reported incidence is 0.2% to less than 0.01% and is best avoided by careful visualization of the ductal structures.
For this trial, both the transgastric and the transvaginal approaches have included the use of a 5-mm puncture in the umbilicus so that a small laparoscope can be used to monitor the procedure for optimum safety and visualization.
Other sites currently participating in the trial include Yale University, New Haven, Conn.; Ohio State University, Columbus; Northwestern University, Chicago; and the Oregon Clinic, Portland.
Patient Inclusion/Exclusion
To be accepted for the trial, patients must be between the ages of 18 and 75 years and present with a diagnosis of benign gallstone disease requiring elective cholecystectomy. Men will be randomized to either transgastric NOTES or laparoscopic surgery, and women will be randomized to all three options.
Patients cannot be obese (BMI greater than 35 kg/m2) or have comorbidities greater than ASA P1 or P2. Patients must not have chronic renal failure, chronic liver disease, or presumed gallbladder malignancy, nor can they be on anticoagulation drugs other than once-daily aspirin.
Previous Research/Trials
Cholecystectomy is the most common procedure for which a NOTES approach has currently been applied, and researchers have used both a transgastric and transvaginal approaches in previous exploratory trials in the United States and other countries. Considerable research has been conducted to improve methods of visualization, hybrid procedures, and duct ligation methods and instrumentation in an effort to minimize potential complications.
About NOSCAR
The trial is sponsored by the NOSCAR, which is a collaboration of the American Society for Gastrointestinal Endoscopy (ASGE) and the Society of American Gastrointestinal and Endoscopic Surgeons* (SAGES). Through NOSCAR, surgeons and interventional endoscopists are working together to investigate the therapeutic potential of natural orifice translumenal endoscopic surgery through funding of basic research and clinical trials.
NOSCAR receives funding from a variety of industry sources for its basic research studies. This clinical trial is being supported by continuing research commitments from industry partners, Ethicon Endo-Surgery, Olympus America, Covidien, Stryker Medical, and KARL STORZ Endoscopy-America, as well as ASGE directly, according to a NOSCAR statement.
Inquiries about the trial can be directed to Barbara Connell.
* Correction, 8/11/2011: The original version of this article included an incorrect name for the Society of American Gastrointestinal and Endoscopic Surgeons. This version has been updated.
In the ongoing effort to limit the physical impact of surgery on the human body, researchers are taking minimally invasive gallbladder removal to the next logical step. Instead of removing the organ through the abdominal wall, surgeons in the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR) are performing cholecystectomy through the mouth or the vagina.
Earlier this year, transoral and transvaginal cholecystectomies were performed by Dr. Santiago Horgan and Dr. John Romanelli, respectively, as part of a new clinical trial designed to provide the first multicenter, prospective, randomized, controlled, noninferiority evaluation of these two natural orifice methods with the laparoscopic approach.
Cholecystectomy, usually for benign gallstone disease, is one of the most common surgical procedures; roughly 750,000 such operations are performed annually in the United States. The goal of natural orifice surgery is to eliminate the need to pierce the abdominal wall during gallbladder removal, thereby lessening the risk of complications and additional pain.
About the Trial
The trial (NCT01171027) was announced in July 2010 and began this year. It has an estimated enrollment of approximately 150 patients. The primary outcome measure is to assess the safety and efficacy of transgastric and transvaginal cholecystectomy, compared with conventional laparoscopic cholecystectomy up to 4 weeks after surgery.
The secondary outcome is to assess pain associated with the oral and the vaginal techniques, as compared with conventional laparoscopic cholecystectomy up to 4 weeks post surgery. Cosmesis associated with transgastric and transvaginal cholecystectomy will also be compared with conventional laparoscopic cholecystectomy as well as costs.
The ultimate goal is to develop the technology further so that the transoral and transgastric approaches would not require any incisions in the abdominal wall. Patient recruitment information is available at the NOSCAR website.
When asked to comment on the trial, Dr. David W. Rattner, chief of the division of gastrointestinal and general surgery, Massachusetts General Hospital, Boston, stated: "It is important to note that while over 5,000 transvaginal cholecystectomies have been performed worldwide, this is the first prospective, randomized study to compare NOTES [natural orifice translumenal endoscopic surgery] approaches to a standard therapy for benign gallstone disease. Principal investigators Dr. Michael Kochman at the University of Pennsylvania, Philadelphia, and Dr. Steven L. Schwaitzberg at Harvard University, Cambridge, initiated all of the hard work to make this important comparative trial happen."
Surgical Approaches
The transgastric cholecystectomy is being performed through the use of an orally introduced flexible endoscope to provide working channels and visualization, followed by a gastrotomy made through the stomach wall, allowing the endoscope to pass into the abdominal cavity. The gallbladder is removed from the abdominal cavity into the stomach and ultimately out of the mouth.
The first such operation in the study was performed by Dr. Horgan of the University of California, San Diego. The transvaginal approach is being performed using a posterior colpotomy and a flexible endoscope instead of a conventional laparoscope. The gallbladder is removed from the vaginal incision. The first such operation in the study was performed by Dr. Romanelli of the Baystate Medical Center in Springfield, Mass.
Both approaches are being compared with a version of traditional laparoscopic cholecystectomy in which a laparoscope is introduced through a 1-cm incision in or around the umbilicus, usually with three additional 0.5- to 1.0-cm incisions employed for surgical instrumentation. The gallbladder is typically removed through the umbilical port. According to the clinical trial summary, "the most devastating complication is injury to the major bile ducts." The reported incidence is 0.2% to less than 0.01% and is best avoided by careful visualization of the ductal structures.
For this trial, both the transgastric and the transvaginal approaches have included the use of a 5-mm puncture in the umbilicus so that a small laparoscope can be used to monitor the procedure for optimum safety and visualization.
Other sites currently participating in the trial include Yale University, New Haven, Conn.; Ohio State University, Columbus; Northwestern University, Chicago; and the Oregon Clinic, Portland.
Patient Inclusion/Exclusion
To be accepted for the trial, patients must be between the ages of 18 and 75 years and present with a diagnosis of benign gallstone disease requiring elective cholecystectomy. Men will be randomized to either transgastric NOTES or laparoscopic surgery, and women will be randomized to all three options.
Patients cannot be obese (BMI greater than 35 kg/m2) or have comorbidities greater than ASA P1 or P2. Patients must not have chronic renal failure, chronic liver disease, or presumed gallbladder malignancy, nor can they be on anticoagulation drugs other than once-daily aspirin.
Previous Research/Trials
Cholecystectomy is the most common procedure for which a NOTES approach has currently been applied, and researchers have used both a transgastric and transvaginal approaches in previous exploratory trials in the United States and other countries. Considerable research has been conducted to improve methods of visualization, hybrid procedures, and duct ligation methods and instrumentation in an effort to minimize potential complications.
About NOSCAR
The trial is sponsored by the NOSCAR, which is a collaboration of the American Society for Gastrointestinal Endoscopy (ASGE) and the Society of American Gastrointestinal and Endoscopic Surgeons* (SAGES). Through NOSCAR, surgeons and interventional endoscopists are working together to investigate the therapeutic potential of natural orifice translumenal endoscopic surgery through funding of basic research and clinical trials.
NOSCAR receives funding from a variety of industry sources for its basic research studies. This clinical trial is being supported by continuing research commitments from industry partners, Ethicon Endo-Surgery, Olympus America, Covidien, Stryker Medical, and KARL STORZ Endoscopy-America, as well as ASGE directly, according to a NOSCAR statement.
Inquiries about the trial can be directed to Barbara Connell.
* Correction, 8/11/2011: The original version of this article included an incorrect name for the Society of American Gastrointestinal and Endoscopic Surgeons. This version has been updated.
In the ongoing effort to limit the physical impact of surgery on the human body, researchers are taking minimally invasive gallbladder removal to the next logical step. Instead of removing the organ through the abdominal wall, surgeons in the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR) are performing cholecystectomy through the mouth or the vagina.
Earlier this year, transoral and transvaginal cholecystectomies were performed by Dr. Santiago Horgan and Dr. John Romanelli, respectively, as part of a new clinical trial designed to provide the first multicenter, prospective, randomized, controlled, noninferiority evaluation of these two natural orifice methods with the laparoscopic approach.
Cholecystectomy, usually for benign gallstone disease, is one of the most common surgical procedures; roughly 750,000 such operations are performed annually in the United States. The goal of natural orifice surgery is to eliminate the need to pierce the abdominal wall during gallbladder removal, thereby lessening the risk of complications and additional pain.
About the Trial
The trial (NCT01171027) was announced in July 2010 and began this year. It has an estimated enrollment of approximately 150 patients. The primary outcome measure is to assess the safety and efficacy of transgastric and transvaginal cholecystectomy, compared with conventional laparoscopic cholecystectomy up to 4 weeks after surgery.
The secondary outcome is to assess pain associated with the oral and the vaginal techniques, as compared with conventional laparoscopic cholecystectomy up to 4 weeks post surgery. Cosmesis associated with transgastric and transvaginal cholecystectomy will also be compared with conventional laparoscopic cholecystectomy as well as costs.
The ultimate goal is to develop the technology further so that the transoral and transgastric approaches would not require any incisions in the abdominal wall. Patient recruitment information is available at the NOSCAR website.
When asked to comment on the trial, Dr. David W. Rattner, chief of the division of gastrointestinal and general surgery, Massachusetts General Hospital, Boston, stated: "It is important to note that while over 5,000 transvaginal cholecystectomies have been performed worldwide, this is the first prospective, randomized study to compare NOTES [natural orifice translumenal endoscopic surgery] approaches to a standard therapy for benign gallstone disease. Principal investigators Dr. Michael Kochman at the University of Pennsylvania, Philadelphia, and Dr. Steven L. Schwaitzberg at Harvard University, Cambridge, initiated all of the hard work to make this important comparative trial happen."
Surgical Approaches
The transgastric cholecystectomy is being performed through the use of an orally introduced flexible endoscope to provide working channels and visualization, followed by a gastrotomy made through the stomach wall, allowing the endoscope to pass into the abdominal cavity. The gallbladder is removed from the abdominal cavity into the stomach and ultimately out of the mouth.
The first such operation in the study was performed by Dr. Horgan of the University of California, San Diego. The transvaginal approach is being performed using a posterior colpotomy and a flexible endoscope instead of a conventional laparoscope. The gallbladder is removed from the vaginal incision. The first such operation in the study was performed by Dr. Romanelli of the Baystate Medical Center in Springfield, Mass.
Both approaches are being compared with a version of traditional laparoscopic cholecystectomy in which a laparoscope is introduced through a 1-cm incision in or around the umbilicus, usually with three additional 0.5- to 1.0-cm incisions employed for surgical instrumentation. The gallbladder is typically removed through the umbilical port. According to the clinical trial summary, "the most devastating complication is injury to the major bile ducts." The reported incidence is 0.2% to less than 0.01% and is best avoided by careful visualization of the ductal structures.
For this trial, both the transgastric and the transvaginal approaches have included the use of a 5-mm puncture in the umbilicus so that a small laparoscope can be used to monitor the procedure for optimum safety and visualization.
Other sites currently participating in the trial include Yale University, New Haven, Conn.; Ohio State University, Columbus; Northwestern University, Chicago; and the Oregon Clinic, Portland.
Patient Inclusion/Exclusion
To be accepted for the trial, patients must be between the ages of 18 and 75 years and present with a diagnosis of benign gallstone disease requiring elective cholecystectomy. Men will be randomized to either transgastric NOTES or laparoscopic surgery, and women will be randomized to all three options.
Patients cannot be obese (BMI greater than 35 kg/m2) or have comorbidities greater than ASA P1 or P2. Patients must not have chronic renal failure, chronic liver disease, or presumed gallbladder malignancy, nor can they be on anticoagulation drugs other than once-daily aspirin.
Previous Research/Trials
Cholecystectomy is the most common procedure for which a NOTES approach has currently been applied, and researchers have used both a transgastric and transvaginal approaches in previous exploratory trials in the United States and other countries. Considerable research has been conducted to improve methods of visualization, hybrid procedures, and duct ligation methods and instrumentation in an effort to minimize potential complications.
About NOSCAR
The trial is sponsored by the NOSCAR, which is a collaboration of the American Society for Gastrointestinal Endoscopy (ASGE) and the Society of American Gastrointestinal and Endoscopic Surgeons* (SAGES). Through NOSCAR, surgeons and interventional endoscopists are working together to investigate the therapeutic potential of natural orifice translumenal endoscopic surgery through funding of basic research and clinical trials.
NOSCAR receives funding from a variety of industry sources for its basic research studies. This clinical trial is being supported by continuing research commitments from industry partners, Ethicon Endo-Surgery, Olympus America, Covidien, Stryker Medical, and KARL STORZ Endoscopy-America, as well as ASGE directly, according to a NOSCAR statement.
Inquiries about the trial can be directed to Barbara Connell.
* Correction, 8/11/2011: The original version of this article included an incorrect name for the Society of American Gastrointestinal and Endoscopic Surgeons. This version has been updated.
Benefits of Perioperative Statins Confirmed
Major Finding: Perioperative statin use was associated with a significant reduction of perioperative cardiovascular events (HR, 0.55) and improved long-term outcome (HR, 0.59).
Data Source: A further analysis of 497 patients in the randomized, double-blind, DECREASE III trial.
Disclosures: Dr. Schouten stated that he had nothing to disclose.
CHICAGO – Results from a follow-up analysis of patients in the randomized, double-blind DECREASE III trial showed that there is an apparent “legacy” effect of perioperative statin therapy, resulting in improved long-term survival, compared with statin initiation after a patient undergoes vascular surgery.
Ischemic cardiac events are a major cause of perioperative morbidity and mortality in noncardiac surgery, with an estimated 10%–40% of perioperative deaths ascribed to myocardial infarction, according to the original report by Dr. Don Poldermans and the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) III researchers. Results of the original DECREASE III study showed that in high-risk patients who undergo major vascular surgery, fluvastatin XL reduced myocardial ischemia and the combined end point of cardiovascular death and MI.
Dr. Olaf Schouten from the Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues further analyzed of the DECREASE III population, examining 497 patients who were randomized to placebo (247 patients) or fluvastatin (250) in the double-blinded trial.
The patients had been started on treatment a median of 34 days prior to surgery. At the end of the DECREASE III study period (30 days after surgery), all patients were prescribed lifelong statins as recommended by current guidelines. The current study relied on all-cause death data obtained from a civil service registry for a median follow-up of 4.8 years, during which time 129 patients died.
Perioperative statin use was linked with a significant reduction of perioperative cardiovascular events (hazard ratio, 0.55), Dr. Schouten said at the meeting. In a multivariate analysis that adjusted for possible confounders including cardiovascular risk factors, type and site of vascular surgery, and age, preoperative statin initiation was still significantly associated with an improved long-term outcome (HR, 0.59).
“This 'legacy' effect might be due to the prevention of perioperative myocardial damage, as patients with myocardial damage had a significantly higher risk of death during follow-up,” Dr. Schouten said.
“The main message of our study is that all vascular surgery patients eligible for statin therapy [with no contraindications] should be prescribed statins at the first, preoperative, and outpatient clinic visit,” said Dr. Schouten in an interview. He pointed out that statin therapy is safe in the perioperative period, with no significant side effects; it improves perioperative cardiac outcome; and it is associated with a long-term survival benefit.
Patients eligible for statin therapy should get statins at the first, preoperative, and outpatient clinic visit.
Source Dr. schouten
Major Finding: Perioperative statin use was associated with a significant reduction of perioperative cardiovascular events (HR, 0.55) and improved long-term outcome (HR, 0.59).
Data Source: A further analysis of 497 patients in the randomized, double-blind, DECREASE III trial.
Disclosures: Dr. Schouten stated that he had nothing to disclose.
CHICAGO – Results from a follow-up analysis of patients in the randomized, double-blind DECREASE III trial showed that there is an apparent “legacy” effect of perioperative statin therapy, resulting in improved long-term survival, compared with statin initiation after a patient undergoes vascular surgery.
Ischemic cardiac events are a major cause of perioperative morbidity and mortality in noncardiac surgery, with an estimated 10%–40% of perioperative deaths ascribed to myocardial infarction, according to the original report by Dr. Don Poldermans and the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) III researchers. Results of the original DECREASE III study showed that in high-risk patients who undergo major vascular surgery, fluvastatin XL reduced myocardial ischemia and the combined end point of cardiovascular death and MI.
Dr. Olaf Schouten from the Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues further analyzed of the DECREASE III population, examining 497 patients who were randomized to placebo (247 patients) or fluvastatin (250) in the double-blinded trial.
The patients had been started on treatment a median of 34 days prior to surgery. At the end of the DECREASE III study period (30 days after surgery), all patients were prescribed lifelong statins as recommended by current guidelines. The current study relied on all-cause death data obtained from a civil service registry for a median follow-up of 4.8 years, during which time 129 patients died.
Perioperative statin use was linked with a significant reduction of perioperative cardiovascular events (hazard ratio, 0.55), Dr. Schouten said at the meeting. In a multivariate analysis that adjusted for possible confounders including cardiovascular risk factors, type and site of vascular surgery, and age, preoperative statin initiation was still significantly associated with an improved long-term outcome (HR, 0.59).
“This 'legacy' effect might be due to the prevention of perioperative myocardial damage, as patients with myocardial damage had a significantly higher risk of death during follow-up,” Dr. Schouten said.
“The main message of our study is that all vascular surgery patients eligible for statin therapy [with no contraindications] should be prescribed statins at the first, preoperative, and outpatient clinic visit,” said Dr. Schouten in an interview. He pointed out that statin therapy is safe in the perioperative period, with no significant side effects; it improves perioperative cardiac outcome; and it is associated with a long-term survival benefit.
Patients eligible for statin therapy should get statins at the first, preoperative, and outpatient clinic visit.
Source Dr. schouten
Major Finding: Perioperative statin use was associated with a significant reduction of perioperative cardiovascular events (HR, 0.55) and improved long-term outcome (HR, 0.59).
Data Source: A further analysis of 497 patients in the randomized, double-blind, DECREASE III trial.
Disclosures: Dr. Schouten stated that he had nothing to disclose.
CHICAGO – Results from a follow-up analysis of patients in the randomized, double-blind DECREASE III trial showed that there is an apparent “legacy” effect of perioperative statin therapy, resulting in improved long-term survival, compared with statin initiation after a patient undergoes vascular surgery.
Ischemic cardiac events are a major cause of perioperative morbidity and mortality in noncardiac surgery, with an estimated 10%–40% of perioperative deaths ascribed to myocardial infarction, according to the original report by Dr. Don Poldermans and the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) III researchers. Results of the original DECREASE III study showed that in high-risk patients who undergo major vascular surgery, fluvastatin XL reduced myocardial ischemia and the combined end point of cardiovascular death and MI.
Dr. Olaf Schouten from the Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues further analyzed of the DECREASE III population, examining 497 patients who were randomized to placebo (247 patients) or fluvastatin (250) in the double-blinded trial.
The patients had been started on treatment a median of 34 days prior to surgery. At the end of the DECREASE III study period (30 days after surgery), all patients were prescribed lifelong statins as recommended by current guidelines. The current study relied on all-cause death data obtained from a civil service registry for a median follow-up of 4.8 years, during which time 129 patients died.
Perioperative statin use was linked with a significant reduction of perioperative cardiovascular events (hazard ratio, 0.55), Dr. Schouten said at the meeting. In a multivariate analysis that adjusted for possible confounders including cardiovascular risk factors, type and site of vascular surgery, and age, preoperative statin initiation was still significantly associated with an improved long-term outcome (HR, 0.59).
“This 'legacy' effect might be due to the prevention of perioperative myocardial damage, as patients with myocardial damage had a significantly higher risk of death during follow-up,” Dr. Schouten said.
“The main message of our study is that all vascular surgery patients eligible for statin therapy [with no contraindications] should be prescribed statins at the first, preoperative, and outpatient clinic visit,” said Dr. Schouten in an interview. He pointed out that statin therapy is safe in the perioperative period, with no significant side effects; it improves perioperative cardiac outcome; and it is associated with a long-term survival benefit.
Patients eligible for statin therapy should get statins at the first, preoperative, and outpatient clinic visit.
Source Dr. schouten
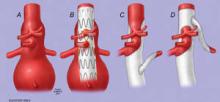
Aneurysm Complexity Influences Outcome
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., found that open complex abdominal aortic aneurysm (cAAA) repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies.
They reviewed the outcomes of a series of 461 patients undergoing open cAAA repair from 2000 to 2010, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator.
A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%.
Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA).
Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but the risk of complications and mortality increases with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., found that open complex abdominal aortic aneurysm (cAAA) repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies.
They reviewed the outcomes of a series of 461 patients undergoing open cAAA repair from 2000 to 2010, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator.
A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%.
Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA).
Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but the risk of complications and mortality increases with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., found that open complex abdominal aortic aneurysm (cAAA) repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies.
They reviewed the outcomes of a series of 461 patients undergoing open cAAA repair from 2000 to 2010, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator.
A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%.
Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA).
Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but the risk of complications and mortality increases with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
Controversy Over Study Questioning EVAR Safety
A study on the potential risks of endovascular repair of abdominal aortic aneurysms used outside the guidelines has caused controversy. The analysis, published in Circulation, found a high incidence of aneurysm sac enlargement after stenting, which the authors tied to an increased risk of aneurysm rupture.
In an accompanying editorial, Dr. Richard P. Cambria contrasted the authors' results with the demonstrated safety of EVAR as seen in clinical trials and practice, and he proposed several trial-related factors to explain the discrepancy.
In the study, Dr. Andres Schanzer of the University of Massachusetts, and his colleagues at the Harvard School of Public Health, Boston, and the Cleveland Clinic Foundation conducted a retrospective analysis of EVAR patients in M2S Inc.'s database.
M2S served as the core imaging laboratory for several large aneurysm management trials and provides these services to public and private academic hospitals globally. The study population consisted of 10,228 patients who underwent EVAR for AAA repair in 1999-2008, and who had a baseline CT scan before EVAR and at least one follow-up CT scan. The patients were primarily men (84.1%) with a mean age of 73.9 years.
They concluded that the majority of patients receiving endovascular repair for an aortic abdominal aneurysm failed to meet criteria for intervention as opposed to surveillance, and that more than 58% of these patients failed to meet conservative criteria for device use based on manufacturer instructions, according to a retrospective database study.
In addition, at 5 years post EVAR, 41% of the patients showed aneurysm sac enlargement, an outcome that the researchers considered a surrogate for potential aneurysm rupture, according to their report now published in print in the June 21 issue of Circulation.
The online "ahead-of-print edition" was summarized in the May Vascular Specialist (Vol 7 No.3 p.1).
In an editorial commentary on the article titled "Endovascular Repair of Abdominal Aortic Aneurysms: No Cause for Alarm," Dr. Richard P. Cambria, current president of the Society for Vascular Surgery, presents a critique of several of the points raised by Dr. Schanzer and his colleagues and comes to the overall conclusion that "The concerns raised in the Schanzer et al. report are, in a sense, legitimate, yet the preponderance of evidence has verified the clinical efficacy of EVAR" (Circulation 2011; 123:2782-3). For example, a large longitudinal study of EVAR in Medicare beneficiaries showed late AAA rupture after EVAR to be a rare event (1.8% at 4 years).
Dr. Cambria took issue with several key points in the article.
Perhaps the most import was the fact that the "alarmingly high" incidence of post-EVAR sac enlargement (17% at 3 years and 41% at 5 years) seen by Dr. Schanzer's group was dramatically higher than seen in other studies and went in the face of a "surfeit of literature encompassing single center cohort studies, registries/trials, and even randomized trials of EVAR verses open repair for AAA," all of which have verified the clinical effectiveness of EVAR.
Dr. Cambria pointed out that in his own group only 8% of sequentially imaged post-EVAR patients demonstrated sac enlargement at 3 years, and in the Eurostar registry, the figure was in the 10% range. In addition, in device-specific trials the rate of sac enlargement ranged from 5% to 12%.
In addition, the significance of sac enlargement after EVAR in the absence of endoleak has been doubted because of a lack of correlation with clinically significant outcomes.
Dr. Cambria indicated that the probable reason for the much higher overall incidence of sac enlargement seen by the authors as compared to the literature is due to an inherent selection bias caused by the study considering only those patients whose surgeons sought further information post-EVAR using an expensive computed tomography scan, typically only obtained when there is some question about the technical results of the EVAR. "Accordingly the denominator in the Schantzer et al. study is unknown, and herein lies the explanation for their AAA sac enlargement data."
With regard to the high incidence of EVAR performed outside the recommended instructions for use (IFU) reported by the authors, Dr. Cambria indicated that the binary nature in which they addressed the question (as liberal or conservative applications) "tends to overestimate the number of procedures performed outside of IFU." Dr. Cambria also stated that in high-risk (for open repair) patients, surgeons "frequently and intentionally compromise on IFU guidelines simply because that is appropriate clinical decision-making."
In addition, "although a general recommendation to adhere to IFU considerations in EVAR is appropriate, favorable results have been achieved when less stringent criteria are applied in accordance with individual patient considerations."
The issue of inappropriate treatment with EVAR of patients with AAA size under the 5.5 cm threshold was also addressed.
"In my view... the article's tone is overly harsh in its implication that the 60% of EVAR patients treated with AAAs less than or equal to 5.5 cm were incorrectly managed." He pointed out that Society for Vascular Surgery guidelines specifically indicate that a variety of clinical and/or anatomic features (not to mention patient preference) clearly influence the decision to proceed with treatment "particularly for AAAs in the 5.0-cm to 5.5-cm range."
He also pointed out that larger AAAs are less likely to be anatomically suitable for EVAR.
Dr. Schanzen and his colleagues reported that they had no disclosures.
Dr. Cambria reported receiving research support in the context of participation in clinical trials from W.L. Gore, Cook, and Medtronic.
A study on the potential risks of endovascular repair of abdominal aortic aneurysms used outside the guidelines has caused controversy. The analysis, published in Circulation, found a high incidence of aneurysm sac enlargement after stenting, which the authors tied to an increased risk of aneurysm rupture.
In an accompanying editorial, Dr. Richard P. Cambria contrasted the authors' results with the demonstrated safety of EVAR as seen in clinical trials and practice, and he proposed several trial-related factors to explain the discrepancy.
In the study, Dr. Andres Schanzer of the University of Massachusetts, and his colleagues at the Harvard School of Public Health, Boston, and the Cleveland Clinic Foundation conducted a retrospective analysis of EVAR patients in M2S Inc.'s database.
M2S served as the core imaging laboratory for several large aneurysm management trials and provides these services to public and private academic hospitals globally. The study population consisted of 10,228 patients who underwent EVAR for AAA repair in 1999-2008, and who had a baseline CT scan before EVAR and at least one follow-up CT scan. The patients were primarily men (84.1%) with a mean age of 73.9 years.
They concluded that the majority of patients receiving endovascular repair for an aortic abdominal aneurysm failed to meet criteria for intervention as opposed to surveillance, and that more than 58% of these patients failed to meet conservative criteria for device use based on manufacturer instructions, according to a retrospective database study.
In addition, at 5 years post EVAR, 41% of the patients showed aneurysm sac enlargement, an outcome that the researchers considered a surrogate for potential aneurysm rupture, according to their report now published in print in the June 21 issue of Circulation.
The online "ahead-of-print edition" was summarized in the May Vascular Specialist (Vol 7 No.3 p.1).
In an editorial commentary on the article titled "Endovascular Repair of Abdominal Aortic Aneurysms: No Cause for Alarm," Dr. Richard P. Cambria, current president of the Society for Vascular Surgery, presents a critique of several of the points raised by Dr. Schanzer and his colleagues and comes to the overall conclusion that "The concerns raised in the Schanzer et al. report are, in a sense, legitimate, yet the preponderance of evidence has verified the clinical efficacy of EVAR" (Circulation 2011; 123:2782-3). For example, a large longitudinal study of EVAR in Medicare beneficiaries showed late AAA rupture after EVAR to be a rare event (1.8% at 4 years).
Dr. Cambria took issue with several key points in the article.
Perhaps the most import was the fact that the "alarmingly high" incidence of post-EVAR sac enlargement (17% at 3 years and 41% at 5 years) seen by Dr. Schanzer's group was dramatically higher than seen in other studies and went in the face of a "surfeit of literature encompassing single center cohort studies, registries/trials, and even randomized trials of EVAR verses open repair for AAA," all of which have verified the clinical effectiveness of EVAR.
Dr. Cambria pointed out that in his own group only 8% of sequentially imaged post-EVAR patients demonstrated sac enlargement at 3 years, and in the Eurostar registry, the figure was in the 10% range. In addition, in device-specific trials the rate of sac enlargement ranged from 5% to 12%.
In addition, the significance of sac enlargement after EVAR in the absence of endoleak has been doubted because of a lack of correlation with clinically significant outcomes.
Dr. Cambria indicated that the probable reason for the much higher overall incidence of sac enlargement seen by the authors as compared to the literature is due to an inherent selection bias caused by the study considering only those patients whose surgeons sought further information post-EVAR using an expensive computed tomography scan, typically only obtained when there is some question about the technical results of the EVAR. "Accordingly the denominator in the Schantzer et al. study is unknown, and herein lies the explanation for their AAA sac enlargement data."
With regard to the high incidence of EVAR performed outside the recommended instructions for use (IFU) reported by the authors, Dr. Cambria indicated that the binary nature in which they addressed the question (as liberal or conservative applications) "tends to overestimate the number of procedures performed outside of IFU." Dr. Cambria also stated that in high-risk (for open repair) patients, surgeons "frequently and intentionally compromise on IFU guidelines simply because that is appropriate clinical decision-making."
In addition, "although a general recommendation to adhere to IFU considerations in EVAR is appropriate, favorable results have been achieved when less stringent criteria are applied in accordance with individual patient considerations."
The issue of inappropriate treatment with EVAR of patients with AAA size under the 5.5 cm threshold was also addressed.
"In my view... the article's tone is overly harsh in its implication that the 60% of EVAR patients treated with AAAs less than or equal to 5.5 cm were incorrectly managed." He pointed out that Society for Vascular Surgery guidelines specifically indicate that a variety of clinical and/or anatomic features (not to mention patient preference) clearly influence the decision to proceed with treatment "particularly for AAAs in the 5.0-cm to 5.5-cm range."
He also pointed out that larger AAAs are less likely to be anatomically suitable for EVAR.
Dr. Schanzen and his colleagues reported that they had no disclosures.
Dr. Cambria reported receiving research support in the context of participation in clinical trials from W.L. Gore, Cook, and Medtronic.
A study on the potential risks of endovascular repair of abdominal aortic aneurysms used outside the guidelines has caused controversy. The analysis, published in Circulation, found a high incidence of aneurysm sac enlargement after stenting, which the authors tied to an increased risk of aneurysm rupture.
In an accompanying editorial, Dr. Richard P. Cambria contrasted the authors' results with the demonstrated safety of EVAR as seen in clinical trials and practice, and he proposed several trial-related factors to explain the discrepancy.
In the study, Dr. Andres Schanzer of the University of Massachusetts, and his colleagues at the Harvard School of Public Health, Boston, and the Cleveland Clinic Foundation conducted a retrospective analysis of EVAR patients in M2S Inc.'s database.
M2S served as the core imaging laboratory for several large aneurysm management trials and provides these services to public and private academic hospitals globally. The study population consisted of 10,228 patients who underwent EVAR for AAA repair in 1999-2008, and who had a baseline CT scan before EVAR and at least one follow-up CT scan. The patients were primarily men (84.1%) with a mean age of 73.9 years.
They concluded that the majority of patients receiving endovascular repair for an aortic abdominal aneurysm failed to meet criteria for intervention as opposed to surveillance, and that more than 58% of these patients failed to meet conservative criteria for device use based on manufacturer instructions, according to a retrospective database study.
In addition, at 5 years post EVAR, 41% of the patients showed aneurysm sac enlargement, an outcome that the researchers considered a surrogate for potential aneurysm rupture, according to their report now published in print in the June 21 issue of Circulation.
The online "ahead-of-print edition" was summarized in the May Vascular Specialist (Vol 7 No.3 p.1).
In an editorial commentary on the article titled "Endovascular Repair of Abdominal Aortic Aneurysms: No Cause for Alarm," Dr. Richard P. Cambria, current president of the Society for Vascular Surgery, presents a critique of several of the points raised by Dr. Schanzer and his colleagues and comes to the overall conclusion that "The concerns raised in the Schanzer et al. report are, in a sense, legitimate, yet the preponderance of evidence has verified the clinical efficacy of EVAR" (Circulation 2011; 123:2782-3). For example, a large longitudinal study of EVAR in Medicare beneficiaries showed late AAA rupture after EVAR to be a rare event (1.8% at 4 years).
Dr. Cambria took issue with several key points in the article.
Perhaps the most import was the fact that the "alarmingly high" incidence of post-EVAR sac enlargement (17% at 3 years and 41% at 5 years) seen by Dr. Schanzer's group was dramatically higher than seen in other studies and went in the face of a "surfeit of literature encompassing single center cohort studies, registries/trials, and even randomized trials of EVAR verses open repair for AAA," all of which have verified the clinical effectiveness of EVAR.
Dr. Cambria pointed out that in his own group only 8% of sequentially imaged post-EVAR patients demonstrated sac enlargement at 3 years, and in the Eurostar registry, the figure was in the 10% range. In addition, in device-specific trials the rate of sac enlargement ranged from 5% to 12%.
In addition, the significance of sac enlargement after EVAR in the absence of endoleak has been doubted because of a lack of correlation with clinically significant outcomes.
Dr. Cambria indicated that the probable reason for the much higher overall incidence of sac enlargement seen by the authors as compared to the literature is due to an inherent selection bias caused by the study considering only those patients whose surgeons sought further information post-EVAR using an expensive computed tomography scan, typically only obtained when there is some question about the technical results of the EVAR. "Accordingly the denominator in the Schantzer et al. study is unknown, and herein lies the explanation for their AAA sac enlargement data."
With regard to the high incidence of EVAR performed outside the recommended instructions for use (IFU) reported by the authors, Dr. Cambria indicated that the binary nature in which they addressed the question (as liberal or conservative applications) "tends to overestimate the number of procedures performed outside of IFU." Dr. Cambria also stated that in high-risk (for open repair) patients, surgeons "frequently and intentionally compromise on IFU guidelines simply because that is appropriate clinical decision-making."
In addition, "although a general recommendation to adhere to IFU considerations in EVAR is appropriate, favorable results have been achieved when less stringent criteria are applied in accordance with individual patient considerations."
The issue of inappropriate treatment with EVAR of patients with AAA size under the 5.5 cm threshold was also addressed.
"In my view... the article's tone is overly harsh in its implication that the 60% of EVAR patients treated with AAAs less than or equal to 5.5 cm were incorrectly managed." He pointed out that Society for Vascular Surgery guidelines specifically indicate that a variety of clinical and/or anatomic features (not to mention patient preference) clearly influence the decision to proceed with treatment "particularly for AAAs in the 5.0-cm to 5.5-cm range."
He also pointed out that larger AAAs are less likely to be anatomically suitable for EVAR.
Dr. Schanzen and his colleagues reported that they had no disclosures.
Dr. Cambria reported receiving research support in the context of participation in clinical trials from W.L. Gore, Cook, and Medtronic.
Transcatheter Aortic Valve Replacement Gets FDA Panel Nod
GAITHERSBURG, MD. – The first transcatheter aortic valve replacement system being considered for use in the United States was voted for approval by the advisory committee of the Circulatory System Devices Panel of the U.S. Food and Drug Administration in their July 20 meeting.
The panel supported the use of the Edwards Sapien Transcatheter Heart Valve, model 9000TFX, sizes 23 mm and 26 mm, using the Retroflex 3 delivery system for transfemoral delivery for the narrow indication of patients with "severe symptomatic aortic stenosis who have been determined by a cardiac surgeon to be inoperable for open aortic valve replacement," which is considered the standard of care for patients who can be surgically treated. This deliberately restricted patient population represents cohort B of PARTNER (Placement of Aortic Transcatheter Valve Trial), a pivotal randomized study that was used as the basis for the device approval submission.
PARTNER showed "impressive mortality results" and quality of life (QOL) benefits attributable to the valve implantation, compared with a heterogeneously treated control group that received medical therapy and a high proportion of palliative balloon valvuloplasty*, according to FDA clinical reviewer and cardiovascular surgeon, Dr. Julie A. Swain.
The proportion of survival at 1 year was 50.3% for the 179 control patients in PARTNER’s cohort B, and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference, according to Dr. Swain.
The control group represented "standard of care," which the FDA and the panel pointed out was actually a misnomer, as there was indeed no standard of care for these inoperable patients because of a lack of demonstrated effectiveness of any therapy in this population. The natural history of such patients upon onset of symptoms is a rapid decline followed by death, generally within a few years. This is a population that is already at risk for high levels of comorbidity and increased death rate because of their general age and degree of sickness.
However, a key concern expressed by both the FDA and the panel – and even by Dr. Craig R. Smith*, principal investigator of the PARTNER trial, who detailed the study findings during the company’s presentation at the meeting – was the high neurologic event rate (transcient ischemic attack [TIA] and stroke) associated with implantation of the transcatheter valve. This was coupled to a complaint by the FDA of the company’s "post hoc adjunctive definition of stroke" which used a modified Rankin scale score for disability (major vs. minor stroke), which was retrospective, nonvalidated, and largely based on patient self-evaluation, which is known to be poor in stroke victims. There were eight total neurologic events in the control group and 25 in the Sapien group, a significant difference. In the control patients, 7 ischemic/unclassified strokes were determined with 1 hemorrhagic event; in the Sapien group there were 16 ischemic/unclassified strokes, 3 TIAs, 3 intracranial hemorrhages, and 3 hemorrhagic events.
An additional complaint cited the lack of objective neurologic monitoring in the perioperative period, with neurologic events being determined by the judgment of the clinical staff based on symptoms observed. This was coupled to concerns regarding the lack of consistent and defined antithrombotic therapy in the trial.
Other concerns that were raised regarding device safety included the incidence of vascular damage in the transcatheter group (presumed to be the result of operator learning curve and the relatively large size of the delivery catheter, compounded by the complicated pathology of the cardiovascular systems of this severely ill patient population), and the presence of aortic regurgitation, some of which could be determined to be caused by paravalvular flow.
The panel agreed with the necessity of continued postmarketing studies, comprising both the continued follow-up of the original PARTNER cohort B and the use of a specific new postmarketing study focusing on the stroke issue in a roll-out of the device at an additional 75 centers, with 10-20 patients being enrolled in the study at each. The panel also urged that all patients receiving the TAVR (transcatheter aortic valve replacement) device be followed in a national registry, with a strong suggestion that the American College of Cardiology/Society of Thoracic Surgeons registry be considered as one possible means for doing this.
Representatives of three of the professional societies that were most involved in the potential use of TAVR technology spoke in support of the approval of the Edwards Sapien system during the open public hearing section of the meeting: Dr. Augusto D. Pichard* of George Washington University in Washington, who represented the SCAI (Society for Cardiovascular Angiography and Interventions); Dr. David Holmes, president of the ACC; and Dr. Michael Mack, president of the Society of Thoracic Surgeons and director of cardiovascular medicine and research at the Heart Hospital Baylor Plano (Texas) system. The latter two organizations have been intimately collaborating in the development of a potential registry, based on current registry systems, that would track all patients with severe aortic stenosis who receive treatment with medical therapy, balloon valvuloplasty, or TAVR for purposes of long-term comparative effectiveness analysis.
All of the society speakers emphasized that this technology must be delivered by heart teams consisting of cardiothoracic surgeons as well as interventional cardiologists, something that has been considered by many as a unique feature of the PARTNER study design.
In the final deliberation, the panel voted 7-3 that the device was safe, 9-1 that the device was effective, and 9-0 (with 1 abstention) that the benefits of the device outweighed the risks. Key elements that were pointed out by dissenting members were the reversed mortality trend seen in the smaller, randomized, "open access" cohort that was not included in the overall analysis; the fear of indication "creep" outside the narrow patient population as a result of having one surgeon as the deciding individual rather than two, as in the PARTNER protocol; and the persistent problem of stroke as a significant safety factor with the device.
The panel and the FDA were extremely concerned that the use of TAVR remain restricted to the narrow PARTNER cohort B population in the use of this particular device. Although some expect that further results of PARTNER in its other cohorts may lead to the search for broader indications, others may wait for the results of the ongoing PARTNER II, which is testing a newer iteration of the Sapien XT device with a smaller delivery profile that may be an improvement with regard to device implantation–related neurologic and vascular events.
The FDA usually follows the recommendations of its advisory panels, but there is no obligation to do so in terms of final device approval.
Only one panel member, Dr. Jeffrey S. Borer, disclosed a potential conflict regarding financial support received by his institution, Cornell University, New York, from Edwards Lifesciences. Dr. Holmes and Dr. Mack stated that they had no relevant conflicts.
Correction, 7/25/2011: An earlier version of this article stated that patients in the control group of the PARTNER Trial Cohort B received palliative balloon angioplasty; they actually received balloon valvuloplasty. Also, Dr. Craig R. Smith was incorrectly referred to as a company spokesperson. This version has been updated.
Correction, 9/12/2011: An earlier version of this article misstated Dr. Augusto D. Pichard's name.
Using the proposed ACC/STS registry for monitoring TAVR, "we can look at data collection and data standards and make sure that they are uniform, so that everybody talks about and defines stroke in the same way. ... Maybe we would learn about underutilization of this technology by having a national registry that included medically treated patients as well as interventional patients."
|
Team-based care and the use of appropriate facilities are essential. "The bottom line is to provide expert care by expert teams in expert centers for carefully and appropriately selected patients to optimize the elements of this truly transformational technology."
David Holmes, M.D., professor of medicine at the Mayo Clinic, Rochester, Minn., is the current president of the American College of Cardiology. He spoke as a voluntary contributor to the Open Public Hearing portion of the panel meeting as a representative of the ACC. He stated that he had no relevant disclosures.
Using the proposed ACC/STS registry for monitoring TAVR, "we can look at data collection and data standards and make sure that they are uniform, so that everybody talks about and defines stroke in the same way. ... Maybe we would learn about underutilization of this technology by having a national registry that included medically treated patients as well as interventional patients."
|
Team-based care and the use of appropriate facilities are essential. "The bottom line is to provide expert care by expert teams in expert centers for carefully and appropriately selected patients to optimize the elements of this truly transformational technology."
David Holmes, M.D., professor of medicine at the Mayo Clinic, Rochester, Minn., is the current president of the American College of Cardiology. He spoke as a voluntary contributor to the Open Public Hearing portion of the panel meeting as a representative of the ACC. He stated that he had no relevant disclosures.
Using the proposed ACC/STS registry for monitoring TAVR, "we can look at data collection and data standards and make sure that they are uniform, so that everybody talks about and defines stroke in the same way. ... Maybe we would learn about underutilization of this technology by having a national registry that included medically treated patients as well as interventional patients."
|
Team-based care and the use of appropriate facilities are essential. "The bottom line is to provide expert care by expert teams in expert centers for carefully and appropriately selected patients to optimize the elements of this truly transformational technology."
David Holmes, M.D., professor of medicine at the Mayo Clinic, Rochester, Minn., is the current president of the American College of Cardiology. He spoke as a voluntary contributor to the Open Public Hearing portion of the panel meeting as a representative of the ACC. He stated that he had no relevant disclosures.
GAITHERSBURG, MD. – The first transcatheter aortic valve replacement system being considered for use in the United States was voted for approval by the advisory committee of the Circulatory System Devices Panel of the U.S. Food and Drug Administration in their July 20 meeting.
The panel supported the use of the Edwards Sapien Transcatheter Heart Valve, model 9000TFX, sizes 23 mm and 26 mm, using the Retroflex 3 delivery system for transfemoral delivery for the narrow indication of patients with "severe symptomatic aortic stenosis who have been determined by a cardiac surgeon to be inoperable for open aortic valve replacement," which is considered the standard of care for patients who can be surgically treated. This deliberately restricted patient population represents cohort B of PARTNER (Placement of Aortic Transcatheter Valve Trial), a pivotal randomized study that was used as the basis for the device approval submission.
PARTNER showed "impressive mortality results" and quality of life (QOL) benefits attributable to the valve implantation, compared with a heterogeneously treated control group that received medical therapy and a high proportion of palliative balloon valvuloplasty*, according to FDA clinical reviewer and cardiovascular surgeon, Dr. Julie A. Swain.
The proportion of survival at 1 year was 50.3% for the 179 control patients in PARTNER’s cohort B, and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference, according to Dr. Swain.
The control group represented "standard of care," which the FDA and the panel pointed out was actually a misnomer, as there was indeed no standard of care for these inoperable patients because of a lack of demonstrated effectiveness of any therapy in this population. The natural history of such patients upon onset of symptoms is a rapid decline followed by death, generally within a few years. This is a population that is already at risk for high levels of comorbidity and increased death rate because of their general age and degree of sickness.
However, a key concern expressed by both the FDA and the panel – and even by Dr. Craig R. Smith*, principal investigator of the PARTNER trial, who detailed the study findings during the company’s presentation at the meeting – was the high neurologic event rate (transcient ischemic attack [TIA] and stroke) associated with implantation of the transcatheter valve. This was coupled to a complaint by the FDA of the company’s "post hoc adjunctive definition of stroke" which used a modified Rankin scale score for disability (major vs. minor stroke), which was retrospective, nonvalidated, and largely based on patient self-evaluation, which is known to be poor in stroke victims. There were eight total neurologic events in the control group and 25 in the Sapien group, a significant difference. In the control patients, 7 ischemic/unclassified strokes were determined with 1 hemorrhagic event; in the Sapien group there were 16 ischemic/unclassified strokes, 3 TIAs, 3 intracranial hemorrhages, and 3 hemorrhagic events.
An additional complaint cited the lack of objective neurologic monitoring in the perioperative period, with neurologic events being determined by the judgment of the clinical staff based on symptoms observed. This was coupled to concerns regarding the lack of consistent and defined antithrombotic therapy in the trial.
Other concerns that were raised regarding device safety included the incidence of vascular damage in the transcatheter group (presumed to be the result of operator learning curve and the relatively large size of the delivery catheter, compounded by the complicated pathology of the cardiovascular systems of this severely ill patient population), and the presence of aortic regurgitation, some of which could be determined to be caused by paravalvular flow.
The panel agreed with the necessity of continued postmarketing studies, comprising both the continued follow-up of the original PARTNER cohort B and the use of a specific new postmarketing study focusing on the stroke issue in a roll-out of the device at an additional 75 centers, with 10-20 patients being enrolled in the study at each. The panel also urged that all patients receiving the TAVR (transcatheter aortic valve replacement) device be followed in a national registry, with a strong suggestion that the American College of Cardiology/Society of Thoracic Surgeons registry be considered as one possible means for doing this.
Representatives of three of the professional societies that were most involved in the potential use of TAVR technology spoke in support of the approval of the Edwards Sapien system during the open public hearing section of the meeting: Dr. Augusto D. Pichard* of George Washington University in Washington, who represented the SCAI (Society for Cardiovascular Angiography and Interventions); Dr. David Holmes, president of the ACC; and Dr. Michael Mack, president of the Society of Thoracic Surgeons and director of cardiovascular medicine and research at the Heart Hospital Baylor Plano (Texas) system. The latter two organizations have been intimately collaborating in the development of a potential registry, based on current registry systems, that would track all patients with severe aortic stenosis who receive treatment with medical therapy, balloon valvuloplasty, or TAVR for purposes of long-term comparative effectiveness analysis.
All of the society speakers emphasized that this technology must be delivered by heart teams consisting of cardiothoracic surgeons as well as interventional cardiologists, something that has been considered by many as a unique feature of the PARTNER study design.
In the final deliberation, the panel voted 7-3 that the device was safe, 9-1 that the device was effective, and 9-0 (with 1 abstention) that the benefits of the device outweighed the risks. Key elements that were pointed out by dissenting members were the reversed mortality trend seen in the smaller, randomized, "open access" cohort that was not included in the overall analysis; the fear of indication "creep" outside the narrow patient population as a result of having one surgeon as the deciding individual rather than two, as in the PARTNER protocol; and the persistent problem of stroke as a significant safety factor with the device.
The panel and the FDA were extremely concerned that the use of TAVR remain restricted to the narrow PARTNER cohort B population in the use of this particular device. Although some expect that further results of PARTNER in its other cohorts may lead to the search for broader indications, others may wait for the results of the ongoing PARTNER II, which is testing a newer iteration of the Sapien XT device with a smaller delivery profile that may be an improvement with regard to device implantation–related neurologic and vascular events.
The FDA usually follows the recommendations of its advisory panels, but there is no obligation to do so in terms of final device approval.
Only one panel member, Dr. Jeffrey S. Borer, disclosed a potential conflict regarding financial support received by his institution, Cornell University, New York, from Edwards Lifesciences. Dr. Holmes and Dr. Mack stated that they had no relevant conflicts.
Correction, 7/25/2011: An earlier version of this article stated that patients in the control group of the PARTNER Trial Cohort B received palliative balloon angioplasty; they actually received balloon valvuloplasty. Also, Dr. Craig R. Smith was incorrectly referred to as a company spokesperson. This version has been updated.
Correction, 9/12/2011: An earlier version of this article misstated Dr. Augusto D. Pichard's name.
GAITHERSBURG, MD. – The first transcatheter aortic valve replacement system being considered for use in the United States was voted for approval by the advisory committee of the Circulatory System Devices Panel of the U.S. Food and Drug Administration in their July 20 meeting.
The panel supported the use of the Edwards Sapien Transcatheter Heart Valve, model 9000TFX, sizes 23 mm and 26 mm, using the Retroflex 3 delivery system for transfemoral delivery for the narrow indication of patients with "severe symptomatic aortic stenosis who have been determined by a cardiac surgeon to be inoperable for open aortic valve replacement," which is considered the standard of care for patients who can be surgically treated. This deliberately restricted patient population represents cohort B of PARTNER (Placement of Aortic Transcatheter Valve Trial), a pivotal randomized study that was used as the basis for the device approval submission.
PARTNER showed "impressive mortality results" and quality of life (QOL) benefits attributable to the valve implantation, compared with a heterogeneously treated control group that received medical therapy and a high proportion of palliative balloon valvuloplasty*, according to FDA clinical reviewer and cardiovascular surgeon, Dr. Julie A. Swain.
The proportion of survival at 1 year was 50.3% for the 179 control patients in PARTNER’s cohort B, and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference, according to Dr. Swain.
The control group represented "standard of care," which the FDA and the panel pointed out was actually a misnomer, as there was indeed no standard of care for these inoperable patients because of a lack of demonstrated effectiveness of any therapy in this population. The natural history of such patients upon onset of symptoms is a rapid decline followed by death, generally within a few years. This is a population that is already at risk for high levels of comorbidity and increased death rate because of their general age and degree of sickness.
However, a key concern expressed by both the FDA and the panel – and even by Dr. Craig R. Smith*, principal investigator of the PARTNER trial, who detailed the study findings during the company’s presentation at the meeting – was the high neurologic event rate (transcient ischemic attack [TIA] and stroke) associated with implantation of the transcatheter valve. This was coupled to a complaint by the FDA of the company’s "post hoc adjunctive definition of stroke" which used a modified Rankin scale score for disability (major vs. minor stroke), which was retrospective, nonvalidated, and largely based on patient self-evaluation, which is known to be poor in stroke victims. There were eight total neurologic events in the control group and 25 in the Sapien group, a significant difference. In the control patients, 7 ischemic/unclassified strokes were determined with 1 hemorrhagic event; in the Sapien group there were 16 ischemic/unclassified strokes, 3 TIAs, 3 intracranial hemorrhages, and 3 hemorrhagic events.
An additional complaint cited the lack of objective neurologic monitoring in the perioperative period, with neurologic events being determined by the judgment of the clinical staff based on symptoms observed. This was coupled to concerns regarding the lack of consistent and defined antithrombotic therapy in the trial.
Other concerns that were raised regarding device safety included the incidence of vascular damage in the transcatheter group (presumed to be the result of operator learning curve and the relatively large size of the delivery catheter, compounded by the complicated pathology of the cardiovascular systems of this severely ill patient population), and the presence of aortic regurgitation, some of which could be determined to be caused by paravalvular flow.
The panel agreed with the necessity of continued postmarketing studies, comprising both the continued follow-up of the original PARTNER cohort B and the use of a specific new postmarketing study focusing on the stroke issue in a roll-out of the device at an additional 75 centers, with 10-20 patients being enrolled in the study at each. The panel also urged that all patients receiving the TAVR (transcatheter aortic valve replacement) device be followed in a national registry, with a strong suggestion that the American College of Cardiology/Society of Thoracic Surgeons registry be considered as one possible means for doing this.
Representatives of three of the professional societies that were most involved in the potential use of TAVR technology spoke in support of the approval of the Edwards Sapien system during the open public hearing section of the meeting: Dr. Augusto D. Pichard* of George Washington University in Washington, who represented the SCAI (Society for Cardiovascular Angiography and Interventions); Dr. David Holmes, president of the ACC; and Dr. Michael Mack, president of the Society of Thoracic Surgeons and director of cardiovascular medicine and research at the Heart Hospital Baylor Plano (Texas) system. The latter two organizations have been intimately collaborating in the development of a potential registry, based on current registry systems, that would track all patients with severe aortic stenosis who receive treatment with medical therapy, balloon valvuloplasty, or TAVR for purposes of long-term comparative effectiveness analysis.
All of the society speakers emphasized that this technology must be delivered by heart teams consisting of cardiothoracic surgeons as well as interventional cardiologists, something that has been considered by many as a unique feature of the PARTNER study design.
In the final deliberation, the panel voted 7-3 that the device was safe, 9-1 that the device was effective, and 9-0 (with 1 abstention) that the benefits of the device outweighed the risks. Key elements that were pointed out by dissenting members were the reversed mortality trend seen in the smaller, randomized, "open access" cohort that was not included in the overall analysis; the fear of indication "creep" outside the narrow patient population as a result of having one surgeon as the deciding individual rather than two, as in the PARTNER protocol; and the persistent problem of stroke as a significant safety factor with the device.
The panel and the FDA were extremely concerned that the use of TAVR remain restricted to the narrow PARTNER cohort B population in the use of this particular device. Although some expect that further results of PARTNER in its other cohorts may lead to the search for broader indications, others may wait for the results of the ongoing PARTNER II, which is testing a newer iteration of the Sapien XT device with a smaller delivery profile that may be an improvement with regard to device implantation–related neurologic and vascular events.
The FDA usually follows the recommendations of its advisory panels, but there is no obligation to do so in terms of final device approval.
Only one panel member, Dr. Jeffrey S. Borer, disclosed a potential conflict regarding financial support received by his institution, Cornell University, New York, from Edwards Lifesciences. Dr. Holmes and Dr. Mack stated that they had no relevant conflicts.
Correction, 7/25/2011: An earlier version of this article stated that patients in the control group of the PARTNER Trial Cohort B received palliative balloon angioplasty; they actually received balloon valvuloplasty. Also, Dr. Craig R. Smith was incorrectly referred to as a company spokesperson. This version has been updated.
Correction, 9/12/2011: An earlier version of this article misstated Dr. Augusto D. Pichard's name.
FROM AN FDA CIRCULATORY SYSTEM DEVICES PANEL MEETING
Case Volume Not a Factor in CABG Results
PHILADELPHIA – Outcomes following isolated coronary artery bypass surgery did not differ significantly based on the volume of such procedures that were performed by individual surgeons or at particular institutions in a study of more than 2,000 patients.
In the setting of a university-based, community-hospital, quality-improvement program, excellent surgical results can consistently be obtained even in relatively low-volume programs. Surgical outcomes are not associated with program or surgeon volume, but are directly correlated with focus on quality as manifested by compliance with evidence-based quality standards, according to the study’s lead investigator, Dr. Paul Kurlansky.
He and his colleagues from the Florida Heart Research Institute, Miami, studied 2,218 consecutive patients having isolated CABG from 2007 to 2009 in a university-based quality-improvement program that emphasized involvement of all surgeons in the academic quality endeavor. End points included operative mortality, major morbidity, and National Quality Forum (NQF)-endorsed process measures as defined by the Society of Thoracic Surgeons (STS).
Procedural volume was analyzed as a categorical and a continuous variable using general estimating equations that accounted for clustering effects and were adjusted for STS risk scores, as well as for propensity for operation in a low- vs. high-volume program, Dr. Kurlansky reported at the annual meeting of the American Association for Thoracic Surgery.
The annual program volume ranged from 67 to 292 procedures (median 136), and surgeon volume ranged from 1 to 124 procedures (median 58). Mortality among all hospitals was 0.8% (ranging from 0% to 2.23%); annual observed/expected mortality was 0.41% overall, ranging from 0% to 1.20%.
A comparison of low-volume (less than 200 cases/year) with high-volume centers (at least 200 cases/year) showed no significant difference in mortality (odds ratio 1.08), morbidity (OR 1.34), or any of the medication process measures.
In addition, there was no difference in mortality (OR 1.59), morbidity (OR 1.20), or medication failure (OR 0.57) between high- (at least 87 cases/year) and low-volume surgeons (less than 87 cases).
After adjusting for both STS risk score and for propensity score, the researchers found no association between either hospital or surgeon volume with regard to mortality or morbidity.
Lack of compliance with NQF measures, however, was significantly and highly predictive of morbidity (OR 1.51), regardless of volume, even after adjustment for predicted risk.
The findings indicate that outcome rather than volume is the metric by which cardiac surgical programs should be evaluated, according to Dr. Kurlansky. In addition, meaningful and active academic involvement may represent a new paradigm for the delivery of quality care at the community hospital level, he said.
"The quality process measures, I believe, are only a surrogate of the entire environment created by the academic affiliation, ones that shift the entire focus of the program toward accountability, education, and excellence: open disclosure, discussion of problems, awareness of advances, and striving toward excellence," he said in an interview.
The policy implications of this study are considerable. "The simple administrative approach of a volume threshold (such as that adopted by the Leapfrog group) may be completely inappropriate. Merely adding volume to a mediocre or poor program will only compound the problem, while potentially removing the excellent service provided to the patients of a high-performing, smaller program," he said.
Dr. Kurlansky pointed out that CABG surgery is unique in that it is a very mature, highly practiced, complex surgical procedure.
Dr. Kurlansky reported he had no disclosures relevant to this presentation. ☐
PHILADELPHIA – Outcomes following isolated coronary artery bypass surgery did not differ significantly based on the volume of such procedures that were performed by individual surgeons or at particular institutions in a study of more than 2,000 patients.
In the setting of a university-based, community-hospital, quality-improvement program, excellent surgical results can consistently be obtained even in relatively low-volume programs. Surgical outcomes are not associated with program or surgeon volume, but are directly correlated with focus on quality as manifested by compliance with evidence-based quality standards, according to the study’s lead investigator, Dr. Paul Kurlansky.
He and his colleagues from the Florida Heart Research Institute, Miami, studied 2,218 consecutive patients having isolated CABG from 2007 to 2009 in a university-based quality-improvement program that emphasized involvement of all surgeons in the academic quality endeavor. End points included operative mortality, major morbidity, and National Quality Forum (NQF)-endorsed process measures as defined by the Society of Thoracic Surgeons (STS).
Procedural volume was analyzed as a categorical and a continuous variable using general estimating equations that accounted for clustering effects and were adjusted for STS risk scores, as well as for propensity for operation in a low- vs. high-volume program, Dr. Kurlansky reported at the annual meeting of the American Association for Thoracic Surgery.
The annual program volume ranged from 67 to 292 procedures (median 136), and surgeon volume ranged from 1 to 124 procedures (median 58). Mortality among all hospitals was 0.8% (ranging from 0% to 2.23%); annual observed/expected mortality was 0.41% overall, ranging from 0% to 1.20%.
A comparison of low-volume (less than 200 cases/year) with high-volume centers (at least 200 cases/year) showed no significant difference in mortality (odds ratio 1.08), morbidity (OR 1.34), or any of the medication process measures.
In addition, there was no difference in mortality (OR 1.59), morbidity (OR 1.20), or medication failure (OR 0.57) between high- (at least 87 cases/year) and low-volume surgeons (less than 87 cases).
After adjusting for both STS risk score and for propensity score, the researchers found no association between either hospital or surgeon volume with regard to mortality or morbidity.
Lack of compliance with NQF measures, however, was significantly and highly predictive of morbidity (OR 1.51), regardless of volume, even after adjustment for predicted risk.
The findings indicate that outcome rather than volume is the metric by which cardiac surgical programs should be evaluated, according to Dr. Kurlansky. In addition, meaningful and active academic involvement may represent a new paradigm for the delivery of quality care at the community hospital level, he said.
"The quality process measures, I believe, are only a surrogate of the entire environment created by the academic affiliation, ones that shift the entire focus of the program toward accountability, education, and excellence: open disclosure, discussion of problems, awareness of advances, and striving toward excellence," he said in an interview.
The policy implications of this study are considerable. "The simple administrative approach of a volume threshold (such as that adopted by the Leapfrog group) may be completely inappropriate. Merely adding volume to a mediocre or poor program will only compound the problem, while potentially removing the excellent service provided to the patients of a high-performing, smaller program," he said.
Dr. Kurlansky pointed out that CABG surgery is unique in that it is a very mature, highly practiced, complex surgical procedure.
Dr. Kurlansky reported he had no disclosures relevant to this presentation. ☐
PHILADELPHIA – Outcomes following isolated coronary artery bypass surgery did not differ significantly based on the volume of such procedures that were performed by individual surgeons or at particular institutions in a study of more than 2,000 patients.
In the setting of a university-based, community-hospital, quality-improvement program, excellent surgical results can consistently be obtained even in relatively low-volume programs. Surgical outcomes are not associated with program or surgeon volume, but are directly correlated with focus on quality as manifested by compliance with evidence-based quality standards, according to the study’s lead investigator, Dr. Paul Kurlansky.
He and his colleagues from the Florida Heart Research Institute, Miami, studied 2,218 consecutive patients having isolated CABG from 2007 to 2009 in a university-based quality-improvement program that emphasized involvement of all surgeons in the academic quality endeavor. End points included operative mortality, major morbidity, and National Quality Forum (NQF)-endorsed process measures as defined by the Society of Thoracic Surgeons (STS).
Procedural volume was analyzed as a categorical and a continuous variable using general estimating equations that accounted for clustering effects and were adjusted for STS risk scores, as well as for propensity for operation in a low- vs. high-volume program, Dr. Kurlansky reported at the annual meeting of the American Association for Thoracic Surgery.
The annual program volume ranged from 67 to 292 procedures (median 136), and surgeon volume ranged from 1 to 124 procedures (median 58). Mortality among all hospitals was 0.8% (ranging from 0% to 2.23%); annual observed/expected mortality was 0.41% overall, ranging from 0% to 1.20%.
A comparison of low-volume (less than 200 cases/year) with high-volume centers (at least 200 cases/year) showed no significant difference in mortality (odds ratio 1.08), morbidity (OR 1.34), or any of the medication process measures.
In addition, there was no difference in mortality (OR 1.59), morbidity (OR 1.20), or medication failure (OR 0.57) between high- (at least 87 cases/year) and low-volume surgeons (less than 87 cases).
After adjusting for both STS risk score and for propensity score, the researchers found no association between either hospital or surgeon volume with regard to mortality or morbidity.
Lack of compliance with NQF measures, however, was significantly and highly predictive of morbidity (OR 1.51), regardless of volume, even after adjustment for predicted risk.
The findings indicate that outcome rather than volume is the metric by which cardiac surgical programs should be evaluated, according to Dr. Kurlansky. In addition, meaningful and active academic involvement may represent a new paradigm for the delivery of quality care at the community hospital level, he said.
"The quality process measures, I believe, are only a surrogate of the entire environment created by the academic affiliation, ones that shift the entire focus of the program toward accountability, education, and excellence: open disclosure, discussion of problems, awareness of advances, and striving toward excellence," he said in an interview.
The policy implications of this study are considerable. "The simple administrative approach of a volume threshold (such as that adopted by the Leapfrog group) may be completely inappropriate. Merely adding volume to a mediocre or poor program will only compound the problem, while potentially removing the excellent service provided to the patients of a high-performing, smaller program," he said.
Dr. Kurlansky pointed out that CABG surgery is unique in that it is a very mature, highly practiced, complex surgical procedure.
Dr. Kurlansky reported he had no disclosures relevant to this presentation. ☐
Posttransplant Survival Improved for PAH Patients
Major Finding: In the period 1997–2005, 25% of patients with idiopathic pulmonary arterial hypertension died on the waiting list. None died on the list between 2005 and 2010. After lung transplantation, the 30-day mortality decreased from 24% in the earlier period to 6% in the later period.
Data Source: A review of 2,918 patients at a single institution who were referred for lung transplantation.
Disclosures: Dr. de Perrot reported receiving speaker and teaching honoraria from Actelion.
PHILADELPHIA – Mortality on the waiting list is still a problem, but long-term survival after lung transplantation of patients with pulmonary arterial hypertension has improved significantly over time, a study has shown.
Pulmonary arterial hypertension (PAH) was classified as idiopathic (iPAH) or associated with congenital heart diseases or connective tissue diseases in the study, which was divided into 1997–2004 and 2005–2010 cohorts.
Of 2,918 patients referred to the program from January 1997 to September 2010, 316 (11%) presented with PAH (World Health Organization Group 1). In these patients, PAH was classified as iPAH (123 patients), congenital (77 patients), connective (102 patients), and other (14). The number of referrals was similar between 1997–2004 and 2005–2010. Follow-up was completed until September 2010 for all patients.
Among the 100 PAH patients listed for lung transplantation (LT), 57 had bilateral LT and 22 had heart LT. Eighteen patients on the waiting list died; three are still waiting. Waiting list mortality was higher for patients with connective tissue diseases, Dr. Marc de Perrot said at the meeting. No patient with iPAH has died on the waiting list since 2005; 25% died before that time, he and his associates at Toronto General Hospital found.
After LT, 30-day mortality decreased from 24% in the first cohort to 6% in the second, a significant difference. Ten-year survival was 56% after bilateral LT and 49% after heart LT, a nonsignificant difference. However, 10-year survival was significantly worse for iPAH patients at 42% vs. 70% for the remaining patients (P = .01). Ten-year survival was best for connective tissue disease (69%) and congenital (70%) patients.
“Patients with connective tissue diseases have a high mortality on the waiting list, but enjoy excellent long-term survival after transplant,” Dr. de Perrot concluded.
Major Finding: In the period 1997–2005, 25% of patients with idiopathic pulmonary arterial hypertension died on the waiting list. None died on the list between 2005 and 2010. After lung transplantation, the 30-day mortality decreased from 24% in the earlier period to 6% in the later period.
Data Source: A review of 2,918 patients at a single institution who were referred for lung transplantation.
Disclosures: Dr. de Perrot reported receiving speaker and teaching honoraria from Actelion.
PHILADELPHIA – Mortality on the waiting list is still a problem, but long-term survival after lung transplantation of patients with pulmonary arterial hypertension has improved significantly over time, a study has shown.
Pulmonary arterial hypertension (PAH) was classified as idiopathic (iPAH) or associated with congenital heart diseases or connective tissue diseases in the study, which was divided into 1997–2004 and 2005–2010 cohorts.
Of 2,918 patients referred to the program from January 1997 to September 2010, 316 (11%) presented with PAH (World Health Organization Group 1). In these patients, PAH was classified as iPAH (123 patients), congenital (77 patients), connective (102 patients), and other (14). The number of referrals was similar between 1997–2004 and 2005–2010. Follow-up was completed until September 2010 for all patients.
Among the 100 PAH patients listed for lung transplantation (LT), 57 had bilateral LT and 22 had heart LT. Eighteen patients on the waiting list died; three are still waiting. Waiting list mortality was higher for patients with connective tissue diseases, Dr. Marc de Perrot said at the meeting. No patient with iPAH has died on the waiting list since 2005; 25% died before that time, he and his associates at Toronto General Hospital found.
After LT, 30-day mortality decreased from 24% in the first cohort to 6% in the second, a significant difference. Ten-year survival was 56% after bilateral LT and 49% after heart LT, a nonsignificant difference. However, 10-year survival was significantly worse for iPAH patients at 42% vs. 70% for the remaining patients (P = .01). Ten-year survival was best for connective tissue disease (69%) and congenital (70%) patients.
“Patients with connective tissue diseases have a high mortality on the waiting list, but enjoy excellent long-term survival after transplant,” Dr. de Perrot concluded.
Major Finding: In the period 1997–2005, 25% of patients with idiopathic pulmonary arterial hypertension died on the waiting list. None died on the list between 2005 and 2010. After lung transplantation, the 30-day mortality decreased from 24% in the earlier period to 6% in the later period.
Data Source: A review of 2,918 patients at a single institution who were referred for lung transplantation.
Disclosures: Dr. de Perrot reported receiving speaker and teaching honoraria from Actelion.
PHILADELPHIA – Mortality on the waiting list is still a problem, but long-term survival after lung transplantation of patients with pulmonary arterial hypertension has improved significantly over time, a study has shown.
Pulmonary arterial hypertension (PAH) was classified as idiopathic (iPAH) or associated with congenital heart diseases or connective tissue diseases in the study, which was divided into 1997–2004 and 2005–2010 cohorts.
Of 2,918 patients referred to the program from January 1997 to September 2010, 316 (11%) presented with PAH (World Health Organization Group 1). In these patients, PAH was classified as iPAH (123 patients), congenital (77 patients), connective (102 patients), and other (14). The number of referrals was similar between 1997–2004 and 2005–2010. Follow-up was completed until September 2010 for all patients.
Among the 100 PAH patients listed for lung transplantation (LT), 57 had bilateral LT and 22 had heart LT. Eighteen patients on the waiting list died; three are still waiting. Waiting list mortality was higher for patients with connective tissue diseases, Dr. Marc de Perrot said at the meeting. No patient with iPAH has died on the waiting list since 2005; 25% died before that time, he and his associates at Toronto General Hospital found.
After LT, 30-day mortality decreased from 24% in the first cohort to 6% in the second, a significant difference. Ten-year survival was 56% after bilateral LT and 49% after heart LT, a nonsignificant difference. However, 10-year survival was significantly worse for iPAH patients at 42% vs. 70% for the remaining patients (P = .01). Ten-year survival was best for connective tissue disease (69%) and congenital (70%) patients.
“Patients with connective tissue diseases have a high mortality on the waiting list, but enjoy excellent long-term survival after transplant,” Dr. de Perrot concluded.
Aneurysm Complexity Influences Outcome
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Complex abdominal aortic aneurysm repair can be performed with low mortality (1.3%), but an increasing level of aneurysm complexity was significantly associated with higher morbidity and mortality.
Data Source: A review of outcomes of 461 patients undergoing open cAAA repair from 2000 to 2010.
Disclosures: Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
Aneurysm Complexity Influences Outcome
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
FROM THE VASCULAR ANNUAL MEETING
Aneurysm Complexity Influences Outcome
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
CHICAGO – Fenestrated grafts increasingly are being used for endovascular aneurysm repair of complex abdominal aortic aneurysms, but there are few benchmarks for comparing outcomes achieved with open repair and EVAR in these situations.
Dr. Gustavo S. Oderich and his colleagues at the Mayo Clinic, Rochester, Minn., reviewed the outcomes of a series of 461 patients undergoing open complex abdominal aortic aneurysm (cAAA) repair from 2000 to 2010.
They found that open cAAA repair can be done safely, but there is an increasing risk of complications and mortality with more complex anatomies, Dr. Oderich said at the Vascular Annual Meeting.
In their study, available preoperative digital imaging data were analyzed by a blinded investigator. A centerline of flow was used to define aneurysm extent and to predict the expected number of fenestrations that would have been required to provide 2 cm of proximal seal for a fenestrated EVAR procedure, had one been performed. End points examined were mortality, morbidity, renal function (RF) deterioration, reinterventions, and survival.
Among the 354 male and 107 female patients (mean age, 73 years), the overall operative mortality was 1.3% (6/461). Some level of morbidity occurred in 57% (260) of the patients treated, and it was severe in 20% (91) of the overall patients.
The overall 5-year patient survival rate was 72%, with a freedom from reintervention rate of 90% and a freedom from RF deterioration rate of 84%. Dr. Oderich discussed how the increasing level of aneurysm complexity was significantly associated with greater operative mortality, severe morbidity, and dialysis rates – regardless of whether the complexity was classified by anatomic considerations (from juxtarenal to suprarenal to type IV thoracic aortic aneurysm, TAA) or by the expected number of fenestrations (one through four), with more fenestrations equivalent to increasing complexity.
At 5 years, patient survival ranged from 76% in juxtarenal patients to 62% for type IV TAA, with severe morbidity ranging from 13% (juxtarenal) to 43% (type IV TAA). Similarly, severe morbidity in patients with only one expected fenestration was 0%, but reached 42% in patients who would have an expected number of four fenestrations.
"Open cAAA repair can be performed safely with low overall mortality (1.3%), but a high risk of complications, mortality, and complications increasing with worse anatomic classification and a higher expected number of fenestrations. These data can provide a needed benchmark for comparison with results of fenestrated EVAR," said Dr. Oderich.
Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Complex abdominal aortic aneurysm repair can be performed with low mortality (1.3%), but an increasing level of aneurysm complexity was significantly associated with higher morbidity and mortality.
Data Source: A review of outcomes of 461 patients undergoing open cAAA repair from 2000 to 2010.
Disclosures: Dr. Oderich stated that he received consulting fees and other remuneration from Medtronic and Cook Medical.