User login
The Prince of Surgery: Sir Astley Cooper
Imagine ligating an arterial aneurysm in the early 1800s, when clipper ships were the fastest mode of transport. Thomas Jefferson was still President and not just the face on the $20 bill, and there was no anesthesia beyond plenty of rum or gin, and when sterile operations were more than 70 years in the future.
This was the world of Sir Astley Cooper, dubbed by his compatriots as "The Prince of Surgery," when he performed what he reported as the "first successful operation on the common carotid artery for aneurysm in the year 1808."
Born on August 23, 1768, Cooper received his medical training at Guy’s Hospital in London, where his uncle, William Cooper, was senior surgeon. But his main medical influence was Henry Cline, a student of the famous surgeon, John Hunter. Rather than embracing a career solely as a private practitioner, Cooper focused on teaching and clinical research at Guy’s, where he became senior surgeon in 1800.
Attractive in person, personality, and station (both as surgeon and through the benefit of a wealthy wife), Cooper became the darling of the influential class of his day, with patients such as various Lords, Dukes, and the Prince of Wales, soon to become King George IV, the obese, profligate, and much despised son of and successor to the "mad monarch" who lost the American Revolution.
It, it is ironic, for a man who was considered the premier teaching surgeon of his era, that Cooper had as his most famous student one of the England’s greatest poets – John Keats. In the Keats’ collection there are disparaging notes he took at one of Cooper’s lectures. Vascular surgeons may be more impressed that Cooper taught Valentine Mott, pioneer American surgeon, and one of the first to ligate the innominate artery.
A hallmark of Cooper’s technical approach was extensive animal experimentation, among them, a dog in which he ligated the carotid and vertebral arteries. The animal turned out to be, as Cooper said, "a good house dog."
In 1805 the 44-year-old Mary Edwards presented to Cooper with an aneurysm of the right common carotid artery, which Cooper ligated. She did well initially, but died 16 days later.
Cooper was convince that she would have survived had the aneurysm not been so large initially. So he tried again on June 22, 1808, when Humphrey Humphreys, a 51-year old porter presented with a left carotid artery aneurysm "the size of a walnut. " This was treated by Sir Cooper by means of a double ligation and a division of the common carotid artery. Humphreys survived until 1821. Sir Cooper did the autopsy and found a left-sided cerebral hemorrhage.
According to his report in the first issue of Guy’s Hospital Reports in 1836, Cooper noted that "[the cerebral hemorrhage] of which he died sufficiently attested the freedom of circulation as well as its force in the cerebral vessels on the side of which the carotid had been tied." Valentine Mott witnessed this operation.
That same afternoon as Cooper performed the carotid operation, he also ligated an external iliac artery for a large femoral aneurysm in a patient who survived another 18 years. Cooper performed this same operation nine more times in his career.
In 1813 Cooper achieved the plum position of professor of comparative anatomy at the Royal College of Surgeons.
In 1817 he developed the use of the buried catgut suture in a patient with a popliteal aneurysm, this allowed a completely closed wound, which prevented the fatal hemorrhage which often resulted from the usual procedure in which silk was used to ligate the artery, with the ends of the ligature brought through the wound. Also in1817, he stretched his talents and the technology too far when he ligated just above the aortic bifurcation in an attempt to save a patient, Charles Hutson, with a large ruptured external iliac aneurysm. Hutson died 40 hours after surgery from the failed attempt that occurred more than 100 years before Rudolph Mataas performed the first successful abdominal aortic aneurysm ligation in 1923.
Sir Astley Cooper died on February 12, 1841 from congestive heart failure. A fervent dissectionist to the end, he left detailed instructions on conducting his own postmortem.
Sources and Additional Information
1) Friedman, S. G. A History of Vascular Surgery.Futura Publishing Company, Inc. 1955.
2) Astley Cooper: His Life and Surgical Contributions (J. Hand Surg. 2011;36A:316-20).
3) Thomas Wakely, Astley Cooper, and the Death of George IV (J. R. Soc. Med. 2007;100:314–20).
Imagine ligating an arterial aneurysm in the early 1800s, when clipper ships were the fastest mode of transport. Thomas Jefferson was still President and not just the face on the $20 bill, and there was no anesthesia beyond plenty of rum or gin, and when sterile operations were more than 70 years in the future.
This was the world of Sir Astley Cooper, dubbed by his compatriots as "The Prince of Surgery," when he performed what he reported as the "first successful operation on the common carotid artery for aneurysm in the year 1808."
Born on August 23, 1768, Cooper received his medical training at Guy’s Hospital in London, where his uncle, William Cooper, was senior surgeon. But his main medical influence was Henry Cline, a student of the famous surgeon, John Hunter. Rather than embracing a career solely as a private practitioner, Cooper focused on teaching and clinical research at Guy’s, where he became senior surgeon in 1800.
Attractive in person, personality, and station (both as surgeon and through the benefit of a wealthy wife), Cooper became the darling of the influential class of his day, with patients such as various Lords, Dukes, and the Prince of Wales, soon to become King George IV, the obese, profligate, and much despised son of and successor to the "mad monarch" who lost the American Revolution.
It, it is ironic, for a man who was considered the premier teaching surgeon of his era, that Cooper had as his most famous student one of the England’s greatest poets – John Keats. In the Keats’ collection there are disparaging notes he took at one of Cooper’s lectures. Vascular surgeons may be more impressed that Cooper taught Valentine Mott, pioneer American surgeon, and one of the first to ligate the innominate artery.
A hallmark of Cooper’s technical approach was extensive animal experimentation, among them, a dog in which he ligated the carotid and vertebral arteries. The animal turned out to be, as Cooper said, "a good house dog."
In 1805 the 44-year-old Mary Edwards presented to Cooper with an aneurysm of the right common carotid artery, which Cooper ligated. She did well initially, but died 16 days later.
Cooper was convince that she would have survived had the aneurysm not been so large initially. So he tried again on June 22, 1808, when Humphrey Humphreys, a 51-year old porter presented with a left carotid artery aneurysm "the size of a walnut. " This was treated by Sir Cooper by means of a double ligation and a division of the common carotid artery. Humphreys survived until 1821. Sir Cooper did the autopsy and found a left-sided cerebral hemorrhage.
According to his report in the first issue of Guy’s Hospital Reports in 1836, Cooper noted that "[the cerebral hemorrhage] of which he died sufficiently attested the freedom of circulation as well as its force in the cerebral vessels on the side of which the carotid had been tied." Valentine Mott witnessed this operation.
That same afternoon as Cooper performed the carotid operation, he also ligated an external iliac artery for a large femoral aneurysm in a patient who survived another 18 years. Cooper performed this same operation nine more times in his career.
In 1813 Cooper achieved the plum position of professor of comparative anatomy at the Royal College of Surgeons.
In 1817 he developed the use of the buried catgut suture in a patient with a popliteal aneurysm, this allowed a completely closed wound, which prevented the fatal hemorrhage which often resulted from the usual procedure in which silk was used to ligate the artery, with the ends of the ligature brought through the wound. Also in1817, he stretched his talents and the technology too far when he ligated just above the aortic bifurcation in an attempt to save a patient, Charles Hutson, with a large ruptured external iliac aneurysm. Hutson died 40 hours after surgery from the failed attempt that occurred more than 100 years before Rudolph Mataas performed the first successful abdominal aortic aneurysm ligation in 1923.
Sir Astley Cooper died on February 12, 1841 from congestive heart failure. A fervent dissectionist to the end, he left detailed instructions on conducting his own postmortem.
Sources and Additional Information
1) Friedman, S. G. A History of Vascular Surgery.Futura Publishing Company, Inc. 1955.
2) Astley Cooper: His Life and Surgical Contributions (J. Hand Surg. 2011;36A:316-20).
3) Thomas Wakely, Astley Cooper, and the Death of George IV (J. R. Soc. Med. 2007;100:314–20).
Imagine ligating an arterial aneurysm in the early 1800s, when clipper ships were the fastest mode of transport. Thomas Jefferson was still President and not just the face on the $20 bill, and there was no anesthesia beyond plenty of rum or gin, and when sterile operations were more than 70 years in the future.
This was the world of Sir Astley Cooper, dubbed by his compatriots as "The Prince of Surgery," when he performed what he reported as the "first successful operation on the common carotid artery for aneurysm in the year 1808."
Born on August 23, 1768, Cooper received his medical training at Guy’s Hospital in London, where his uncle, William Cooper, was senior surgeon. But his main medical influence was Henry Cline, a student of the famous surgeon, John Hunter. Rather than embracing a career solely as a private practitioner, Cooper focused on teaching and clinical research at Guy’s, where he became senior surgeon in 1800.
Attractive in person, personality, and station (both as surgeon and through the benefit of a wealthy wife), Cooper became the darling of the influential class of his day, with patients such as various Lords, Dukes, and the Prince of Wales, soon to become King George IV, the obese, profligate, and much despised son of and successor to the "mad monarch" who lost the American Revolution.
It, it is ironic, for a man who was considered the premier teaching surgeon of his era, that Cooper had as his most famous student one of the England’s greatest poets – John Keats. In the Keats’ collection there are disparaging notes he took at one of Cooper’s lectures. Vascular surgeons may be more impressed that Cooper taught Valentine Mott, pioneer American surgeon, and one of the first to ligate the innominate artery.
A hallmark of Cooper’s technical approach was extensive animal experimentation, among them, a dog in which he ligated the carotid and vertebral arteries. The animal turned out to be, as Cooper said, "a good house dog."
In 1805 the 44-year-old Mary Edwards presented to Cooper with an aneurysm of the right common carotid artery, which Cooper ligated. She did well initially, but died 16 days later.
Cooper was convince that she would have survived had the aneurysm not been so large initially. So he tried again on June 22, 1808, when Humphrey Humphreys, a 51-year old porter presented with a left carotid artery aneurysm "the size of a walnut. " This was treated by Sir Cooper by means of a double ligation and a division of the common carotid artery. Humphreys survived until 1821. Sir Cooper did the autopsy and found a left-sided cerebral hemorrhage.
According to his report in the first issue of Guy’s Hospital Reports in 1836, Cooper noted that "[the cerebral hemorrhage] of which he died sufficiently attested the freedom of circulation as well as its force in the cerebral vessels on the side of which the carotid had been tied." Valentine Mott witnessed this operation.
That same afternoon as Cooper performed the carotid operation, he also ligated an external iliac artery for a large femoral aneurysm in a patient who survived another 18 years. Cooper performed this same operation nine more times in his career.
In 1813 Cooper achieved the plum position of professor of comparative anatomy at the Royal College of Surgeons.
In 1817 he developed the use of the buried catgut suture in a patient with a popliteal aneurysm, this allowed a completely closed wound, which prevented the fatal hemorrhage which often resulted from the usual procedure in which silk was used to ligate the artery, with the ends of the ligature brought through the wound. Also in1817, he stretched his talents and the technology too far when he ligated just above the aortic bifurcation in an attempt to save a patient, Charles Hutson, with a large ruptured external iliac aneurysm. Hutson died 40 hours after surgery from the failed attempt that occurred more than 100 years before Rudolph Mataas performed the first successful abdominal aortic aneurysm ligation in 1923.
Sir Astley Cooper died on February 12, 1841 from congestive heart failure. A fervent dissectionist to the end, he left detailed instructions on conducting his own postmortem.
Sources and Additional Information
1) Friedman, S. G. A History of Vascular Surgery.Futura Publishing Company, Inc. 1955.
2) Astley Cooper: His Life and Surgical Contributions (J. Hand Surg. 2011;36A:316-20).
3) Thomas Wakely, Astley Cooper, and the Death of George IV (J. R. Soc. Med. 2007;100:314–20).
No Survival Benefit Seen for EVAR Over Open Surgery for Ruptured AAA
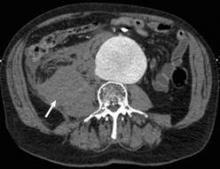
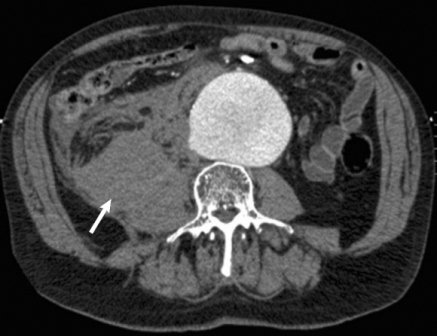
Endovascular repair of ruptured abdominal aortic aneurysms confers no acute or late mortality benefit over open surgery, according to the results of a retrospective study using propensity-score matching by researchers at the University of Pittsburgh Medical Center.
Given these results and the massive systemic change and resources needed to organize and sustain an endovascular program, widespread adoption and routine use of endovascular repair of ruptured AAA (REVAR) should be deferred until there is good evidence of its superiority, according to Dr. Jae-Sung Cho and his colleagues.
Currently, the majority of ruptured AAAs are repaired using open surgery, but there is increasing enthusiasm for REVAR based on results from case reports and single-center series that used a standard REVAR protocol, according to the researchers. Those results are suspect because of the problem of selection bias and limited follow-up, the investigators said.
In an attempt to clarify the situation, Dr. Cho and his colleagues reviewed 312 patients who underwent ruptured abdominal aortic aneurysm (rAAA) repair from January 2001 to November 2010 at the University of Pittsburgh Medical Center (UPMC), a regional tertiary referral hospital with a high volume of open and emergent aortic surgery cases, according to the report in the September issue of the Journal of Vascular Surgery (2012;56:614-19).
A total of 34 patients were excluded owing to prior open or endovascular repair or because they were symptomatic without actual rupture. The remaining 288 patients formed the basis of the analysis: 37 underwent REVAR and 251 were treated with open surgical repair (OSR).
The researchers used propensity score–based matching to reduce the confounding effects of covariate imbalance between the REVAR and OSR as well as potential selection bias. A 1:3 ratio was used in the matching because of the various sample sizes of the REVAR and OSR patients and their distributions of propensity scores, yielding 37 REVAR patients matched to 111 OSR patients for analysis.
The matched cohorts did not significantly differ according to sex (the majority were men: 70.3% of the REVAR group, 62.2% of the OSR group); age (around 75 years in both groups); or history of comorbidities, including chronic obstructive pulmonary disease, cerebrovascular accident, hypertension, peripheral vascular disease, diabetes, smoking, hemodialysis, and coronary artery disease.
The only significant demographic difference between the patient groups was presentation with hemodynamic instability, defined as a systolic blood pressure less than 80 mm Hg. Such patients were more likely to undergo OSR (44%, compared with 24% of patients who underwent REVAR).
The outcomes measured comprised operative mortality (defined as intraoperative, in-hospital, and 30-day mortality), postoperative morbidity, length of stay, and transfusion of blood products. Long-term mortality was determined by evaluating against the Social Security Death Index. Late survival was estimated by Kaplan-Meier methods.
Although operative time and blood replacement were higher with OSR, overall complication rates were not significantly different (54% with REVAR vs. 66% with OSR). However, tracheostomies (21% vs. 3%), myocardial infarction (38% vs. 18%), and acute tubular necrosis (47% vs. 21%) were all significantly higher with OSR.
"Operative mortality rates were similar," according to the authors: 22% with REVAR vs. 32% with OSR (odds ratio, 0.63 for REVAR; 95% confidence interval, 0.24-1.48), and the incidences of secondary interventions for aneurysm- or graft-related complications were identical at 22%.
Kaplan-Meier estimates of 1-, 2-, and 3-year survival rates were not significantly different at 50%, 50%, and 42%, respectively, for REVAR and 54%, 52%, and 47% for OSR (P = .66).
The investigators discussed these results within the scope of several prospective, randomized trials that have been conducted or are currently underway comparing REVAR with OSR. The Nottingham trial and the Amsterdam Acute Aneurysm (AJAX) trial also failed to demonstrate any survival benefits from REVAR. The ongoing IMPROVE trial should be large enough (600 randomized patients) and should be able to address the question of whether or not a REVAR first approach provides a survival benefit over OSR.
Widespread adoption of a REVAR first approach is attractive, according to the researchers, who detail the value of its minimally invasive nature and the lower rates of complications, as demonstrated in their own study.
"However, implementation of such a protocol involves massive systemic changes, such as stocking of endovascular stent grafts and auxiliaries, and around-the-clock availability of skilled endovascular, radiologic, and nursing teams. It is not pragmatic to undertake such systemic and systematic changes in the absence of clear evidence of REVAR’s superiority," they said. For these reasons, no such change was established at the UPMC system, which would represent "a monumental task in a variety of ways."
The limitations the authors pointed out were those inherent to a retrospective study with a relatively small sample size. In addition, there might have been a selection bias on the choice of treatment rendered. Propensity score–based matching can only remove overt bias; unlike randomization, it cannot remove hidden biases.
"There is still equipoise on what the best treatment for ruptured AAA is. The data [in the literature] are conflicting, and many studies showing improved outcomes with REVAR employed it preferentially for patients with favorable anatomy and hemodynamic stability. Preferential REVAR should be deferred until results of prospective, randomized trials are available and predictive factors for its success are identified. Some patients may be harmed by indiscriminate insistence on REVAR," Dr. Cho and his colleagues concluded.
The authors reported that they had no conflicts of interest with regard to their paper.

|
|
[The researchers’] experience indicates that, with expertise in both emergent endovascular aneurysm repair (EVAR) and OSR, the short-term mortality can be equally reduced in both groups, albeit the morbidity of ruptured EVAR is significantly lower. It would also appear that all 37 patients (100%) with ruptured EVAR and only 111 of 241 patients (46%) with ruptured OSR ... underwent propensity score–based analysis. ... I feel obliged to comment that their inability to include the remaining 54% of patients with ruptured OSR has resulted in a cumulative midterm Kaplan-Meier survival analysis that would strongly bias in favor of OSR.
Dr. Manish Mehta is a vascular surgeon with the Albany (N.Y.) Vascular Group. His remarks were part of an invited commentary (J. Vasc. Surg. 2012;56:620). Dr. Mehta did not disclose any potential conflicts of interest.

|
|
[The researchers’] experience indicates that, with expertise in both emergent endovascular aneurysm repair (EVAR) and OSR, the short-term mortality can be equally reduced in both groups, albeit the morbidity of ruptured EVAR is significantly lower. It would also appear that all 37 patients (100%) with ruptured EVAR and only 111 of 241 patients (46%) with ruptured OSR ... underwent propensity score–based analysis. ... I feel obliged to comment that their inability to include the remaining 54% of patients with ruptured OSR has resulted in a cumulative midterm Kaplan-Meier survival analysis that would strongly bias in favor of OSR.
Dr. Manish Mehta is a vascular surgeon with the Albany (N.Y.) Vascular Group. His remarks were part of an invited commentary (J. Vasc. Surg. 2012;56:620). Dr. Mehta did not disclose any potential conflicts of interest.

|
|
[The researchers’] experience indicates that, with expertise in both emergent endovascular aneurysm repair (EVAR) and OSR, the short-term mortality can be equally reduced in both groups, albeit the morbidity of ruptured EVAR is significantly lower. It would also appear that all 37 patients (100%) with ruptured EVAR and only 111 of 241 patients (46%) with ruptured OSR ... underwent propensity score–based analysis. ... I feel obliged to comment that their inability to include the remaining 54% of patients with ruptured OSR has resulted in a cumulative midterm Kaplan-Meier survival analysis that would strongly bias in favor of OSR.
Dr. Manish Mehta is a vascular surgeon with the Albany (N.Y.) Vascular Group. His remarks were part of an invited commentary (J. Vasc. Surg. 2012;56:620). Dr. Mehta did not disclose any potential conflicts of interest.
Endovascular repair of ruptured abdominal aortic aneurysms confers no acute or late mortality benefit over open surgery, according to the results of a retrospective study using propensity-score matching by researchers at the University of Pittsburgh Medical Center.
Given these results and the massive systemic change and resources needed to organize and sustain an endovascular program, widespread adoption and routine use of endovascular repair of ruptured AAA (REVAR) should be deferred until there is good evidence of its superiority, according to Dr. Jae-Sung Cho and his colleagues.
Currently, the majority of ruptured AAAs are repaired using open surgery, but there is increasing enthusiasm for REVAR based on results from case reports and single-center series that used a standard REVAR protocol, according to the researchers. Those results are suspect because of the problem of selection bias and limited follow-up, the investigators said.
In an attempt to clarify the situation, Dr. Cho and his colleagues reviewed 312 patients who underwent ruptured abdominal aortic aneurysm (rAAA) repair from January 2001 to November 2010 at the University of Pittsburgh Medical Center (UPMC), a regional tertiary referral hospital with a high volume of open and emergent aortic surgery cases, according to the report in the September issue of the Journal of Vascular Surgery (2012;56:614-19).
A total of 34 patients were excluded owing to prior open or endovascular repair or because they were symptomatic without actual rupture. The remaining 288 patients formed the basis of the analysis: 37 underwent REVAR and 251 were treated with open surgical repair (OSR).
The researchers used propensity score–based matching to reduce the confounding effects of covariate imbalance between the REVAR and OSR as well as potential selection bias. A 1:3 ratio was used in the matching because of the various sample sizes of the REVAR and OSR patients and their distributions of propensity scores, yielding 37 REVAR patients matched to 111 OSR patients for analysis.
The matched cohorts did not significantly differ according to sex (the majority were men: 70.3% of the REVAR group, 62.2% of the OSR group); age (around 75 years in both groups); or history of comorbidities, including chronic obstructive pulmonary disease, cerebrovascular accident, hypertension, peripheral vascular disease, diabetes, smoking, hemodialysis, and coronary artery disease.
The only significant demographic difference between the patient groups was presentation with hemodynamic instability, defined as a systolic blood pressure less than 80 mm Hg. Such patients were more likely to undergo OSR (44%, compared with 24% of patients who underwent REVAR).
The outcomes measured comprised operative mortality (defined as intraoperative, in-hospital, and 30-day mortality), postoperative morbidity, length of stay, and transfusion of blood products. Long-term mortality was determined by evaluating against the Social Security Death Index. Late survival was estimated by Kaplan-Meier methods.
Although operative time and blood replacement were higher with OSR, overall complication rates were not significantly different (54% with REVAR vs. 66% with OSR). However, tracheostomies (21% vs. 3%), myocardial infarction (38% vs. 18%), and acute tubular necrosis (47% vs. 21%) were all significantly higher with OSR.
"Operative mortality rates were similar," according to the authors: 22% with REVAR vs. 32% with OSR (odds ratio, 0.63 for REVAR; 95% confidence interval, 0.24-1.48), and the incidences of secondary interventions for aneurysm- or graft-related complications were identical at 22%.
Kaplan-Meier estimates of 1-, 2-, and 3-year survival rates were not significantly different at 50%, 50%, and 42%, respectively, for REVAR and 54%, 52%, and 47% for OSR (P = .66).
The investigators discussed these results within the scope of several prospective, randomized trials that have been conducted or are currently underway comparing REVAR with OSR. The Nottingham trial and the Amsterdam Acute Aneurysm (AJAX) trial also failed to demonstrate any survival benefits from REVAR. The ongoing IMPROVE trial should be large enough (600 randomized patients) and should be able to address the question of whether or not a REVAR first approach provides a survival benefit over OSR.
Widespread adoption of a REVAR first approach is attractive, according to the researchers, who detail the value of its minimally invasive nature and the lower rates of complications, as demonstrated in their own study.
"However, implementation of such a protocol involves massive systemic changes, such as stocking of endovascular stent grafts and auxiliaries, and around-the-clock availability of skilled endovascular, radiologic, and nursing teams. It is not pragmatic to undertake such systemic and systematic changes in the absence of clear evidence of REVAR’s superiority," they said. For these reasons, no such change was established at the UPMC system, which would represent "a monumental task in a variety of ways."
The limitations the authors pointed out were those inherent to a retrospective study with a relatively small sample size. In addition, there might have been a selection bias on the choice of treatment rendered. Propensity score–based matching can only remove overt bias; unlike randomization, it cannot remove hidden biases.
"There is still equipoise on what the best treatment for ruptured AAA is. The data [in the literature] are conflicting, and many studies showing improved outcomes with REVAR employed it preferentially for patients with favorable anatomy and hemodynamic stability. Preferential REVAR should be deferred until results of prospective, randomized trials are available and predictive factors for its success are identified. Some patients may be harmed by indiscriminate insistence on REVAR," Dr. Cho and his colleagues concluded.
The authors reported that they had no conflicts of interest with regard to their paper.
Endovascular repair of ruptured abdominal aortic aneurysms confers no acute or late mortality benefit over open surgery, according to the results of a retrospective study using propensity-score matching by researchers at the University of Pittsburgh Medical Center.
Given these results and the massive systemic change and resources needed to organize and sustain an endovascular program, widespread adoption and routine use of endovascular repair of ruptured AAA (REVAR) should be deferred until there is good evidence of its superiority, according to Dr. Jae-Sung Cho and his colleagues.
Currently, the majority of ruptured AAAs are repaired using open surgery, but there is increasing enthusiasm for REVAR based on results from case reports and single-center series that used a standard REVAR protocol, according to the researchers. Those results are suspect because of the problem of selection bias and limited follow-up, the investigators said.
In an attempt to clarify the situation, Dr. Cho and his colleagues reviewed 312 patients who underwent ruptured abdominal aortic aneurysm (rAAA) repair from January 2001 to November 2010 at the University of Pittsburgh Medical Center (UPMC), a regional tertiary referral hospital with a high volume of open and emergent aortic surgery cases, according to the report in the September issue of the Journal of Vascular Surgery (2012;56:614-19).
A total of 34 patients were excluded owing to prior open or endovascular repair or because they were symptomatic without actual rupture. The remaining 288 patients formed the basis of the analysis: 37 underwent REVAR and 251 were treated with open surgical repair (OSR).
The researchers used propensity score–based matching to reduce the confounding effects of covariate imbalance between the REVAR and OSR as well as potential selection bias. A 1:3 ratio was used in the matching because of the various sample sizes of the REVAR and OSR patients and their distributions of propensity scores, yielding 37 REVAR patients matched to 111 OSR patients for analysis.
The matched cohorts did not significantly differ according to sex (the majority were men: 70.3% of the REVAR group, 62.2% of the OSR group); age (around 75 years in both groups); or history of comorbidities, including chronic obstructive pulmonary disease, cerebrovascular accident, hypertension, peripheral vascular disease, diabetes, smoking, hemodialysis, and coronary artery disease.
The only significant demographic difference between the patient groups was presentation with hemodynamic instability, defined as a systolic blood pressure less than 80 mm Hg. Such patients were more likely to undergo OSR (44%, compared with 24% of patients who underwent REVAR).
The outcomes measured comprised operative mortality (defined as intraoperative, in-hospital, and 30-day mortality), postoperative morbidity, length of stay, and transfusion of blood products. Long-term mortality was determined by evaluating against the Social Security Death Index. Late survival was estimated by Kaplan-Meier methods.
Although operative time and blood replacement were higher with OSR, overall complication rates were not significantly different (54% with REVAR vs. 66% with OSR). However, tracheostomies (21% vs. 3%), myocardial infarction (38% vs. 18%), and acute tubular necrosis (47% vs. 21%) were all significantly higher with OSR.
"Operative mortality rates were similar," according to the authors: 22% with REVAR vs. 32% with OSR (odds ratio, 0.63 for REVAR; 95% confidence interval, 0.24-1.48), and the incidences of secondary interventions for aneurysm- or graft-related complications were identical at 22%.
Kaplan-Meier estimates of 1-, 2-, and 3-year survival rates were not significantly different at 50%, 50%, and 42%, respectively, for REVAR and 54%, 52%, and 47% for OSR (P = .66).
The investigators discussed these results within the scope of several prospective, randomized trials that have been conducted or are currently underway comparing REVAR with OSR. The Nottingham trial and the Amsterdam Acute Aneurysm (AJAX) trial also failed to demonstrate any survival benefits from REVAR. The ongoing IMPROVE trial should be large enough (600 randomized patients) and should be able to address the question of whether or not a REVAR first approach provides a survival benefit over OSR.
Widespread adoption of a REVAR first approach is attractive, according to the researchers, who detail the value of its minimally invasive nature and the lower rates of complications, as demonstrated in their own study.
"However, implementation of such a protocol involves massive systemic changes, such as stocking of endovascular stent grafts and auxiliaries, and around-the-clock availability of skilled endovascular, radiologic, and nursing teams. It is not pragmatic to undertake such systemic and systematic changes in the absence of clear evidence of REVAR’s superiority," they said. For these reasons, no such change was established at the UPMC system, which would represent "a monumental task in a variety of ways."
The limitations the authors pointed out were those inherent to a retrospective study with a relatively small sample size. In addition, there might have been a selection bias on the choice of treatment rendered. Propensity score–based matching can only remove overt bias; unlike randomization, it cannot remove hidden biases.
"There is still equipoise on what the best treatment for ruptured AAA is. The data [in the literature] are conflicting, and many studies showing improved outcomes with REVAR employed it preferentially for patients with favorable anatomy and hemodynamic stability. Preferential REVAR should be deferred until results of prospective, randomized trials are available and predictive factors for its success are identified. Some patients may be harmed by indiscriminate insistence on REVAR," Dr. Cho and his colleagues concluded.
The authors reported that they had no conflicts of interest with regard to their paper.
FROM THE JOURNAL OF VASCULAR SURGERY
Major Finding: Kaplan-Meier estimates of 1-, 2-, and 3-year survival rates were not significantly different, at 50%, 50%, and 42%, respectively, for REVAR and 54%, 52%, and 47% for open repair.
Data Source: Outcomes were reviewed for patients in a single center, retrospective, propensity score–matched study comprising 37 patients who underwent REVAR and 251 treated with open surgical repair.
Disclosures: The authors reported that they had no conflicts of interest with regard to their paper.
Assimilating Simulation in Surgical Training: Dainty Morsels or Pig in a Python?
Simulation is an increasing part of surgical training and a critical component in producing the next generation of surgeons – but it is also the focus of questions and controversy, as evidenced by a spate of recent journal articles and by views expressed at a special session of the 2012 Vascular Annual Meeting.
Reasons for the interest in simulation include duty hour restrictions, more complex and automated procedures, and stricter quality assurance programs such as pay for performance – all of which mandate the need to practice and perfect difficult techniques. The push toward simulation is projected to affect not only residents in early training but also established surgeons pursuing Maintenance of Certification (MOC).
Questions seem to have moved beyond simply whether simulation is needed to how much simulation training is needed and how to pay for it. Can simulation training be administered as dainty morsels nibbled over time, or does it need to be swallowed all at once – the pig-in-a-python approach?
It all depends on what level of simulation is necessary. Suturing can be practiced on rubber tubing or on the latest synthetic bioskin products, which mimic multiple skin layers. Arterial procedures can be mimicked by operating on tubing in plastic see-through dummies, or by using robotic simulators to operate on virtual patients generated as three-dimensional CT scans. And the standard of performing simulated operations in the cadaver lab still exists.
Everything ranging from relatively simple, individual technique simulators that cost comparatively little to massive, regional simulation centers that cost hundreds of thousands of dollars are now being used in surgical training. Highly visible examples of what is possible (if you can afford it) are the Goodman Simulation Center at Stanford (Calif.) University, the Stony Brook Medicine Surgical Skills Center at the State University of New York at Stony Brook, the Methodist DeBakey Heart and Vascular Center in Houston, and the University of South Florida Center for Advanced Medical Learning and Simulation in Tampa.
Since 2008, the Accreditation Council for Graduate Medical Education (ACGME) has required, for general surgical training, that resources "include simulation and skills laboratories. These facilities must address acquisition and maintenance of skills with a competency-based method of evaluation." But the extent to which simulation should play a role in training, how much simulation should be required, and how it should be evaluated are subject to debate. The only consensus seems to be that something is needed, and simulation is here to stay, like it or not.
Why Now?
The need for simulation training appears to be driven by several intersecting forces – social and economic problems for which simulation claims to be the solution.
"During the past 100 years, time-based apprentice-type surgical training programs have produced many superb surgeons. However, with restrictions in residency work hours, increasing emphasis on patient safety, and rising costs for training in the operating room, trainees now have fewer opportunities to train in the operating room," according to Dr. Boris Zevin of the University of Toronto and his colleagues.
"The major driving force behind the call to incorporate simulation into surgical training comes from the need to improve patient safety and to shorten the learning curve in the operating room," Dr. Zevin, leader of an international survey that assessed simulation-based training in surgery, and his colleagues wrote in the Journal of the American College of Surgeons (2012 [doi: 10.1016/j.jamcollsurg.2012.05.035]).
In a recent survey, residents in a single surgical simulation training program expressed concern that the need for simulation wasn’t being driven by the health care system or from within the surgical community. Two candid opinions in the survey stated that simulation is "filling this public thirst for there being a uniform, regulated – on a national level – program by which we are all practicing on, basically, objects, before practicing on people," and "a core component of just some way for the health care industry to prove that there are competent people and this is what they are competent in" (Surgery 2012;151:815-21).
Ultimately, one of the strongest pressures to alter resident education may well be financial. According to Dr. John F. Eidt of the University of Arkansas for Medical Sciences, Little Rock, a leading voice for simulation training in vascular surgery education, "The federal government is the primary source of funding for graduate medical education (GME) through supplementation of hospital reimbursement. Recent events threaten to drastically alter GME funding. ... The Medicare Payment Advisory Commission has stated that up to 50% of indirect GME reimbursement is not ‘empirically justified’ by actual hospital costs associated with education," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery.
At the Vascular Annual Meeting, he reiterated his support for simulator training, and discussed the success of the aviation model of simulation in detail.
The Case for Simulation
If adopting simulation is all but inevitable, how best to do it? The dainty morsels vs. pig-in-a-python approach was at the crux of a debate held at the Vascular Annual Meeting session. On the pro-simulation side, Dr. Alan B. Lumsden of the Methodist DeBakey Heart and Vascular Center, Houston, saw simulation as "the wave of the future."
Dr. Lumsden was adamant in saying that the costly, high-fidelity virtual reality systems are not what simulation training for residents should be about. He used CPR dummies as an example of one of the most effective simulators. These cost "about a hundred bucks a pop," compared with some $250,000 systems, he noted, adding that almost every senior vascular surgeon beside him on the podium learned how to do endovascular procedures on foam rubber iliac and aortic stenosis models (including his debate adversary, Dr. Michael S. Makaroun).
"The question is not whether simulation is valid, but the question is whether we as vascular surgeons can figure out how to take what we have learned and learn how to apply that [to simulations]." His major point was that there should be a focus on simulation and how to standardize its use. He indicated that in single tasks, simulation could be made ideal, such that eventually "we should be all doing the procedure in the exact same way," and he gave the example of groin puncture to access a femoral vessel as one good place to start.
Dr. Lumsden also pointed out the critical validity of simulation for team training. He used the example of extracorporeal membrane oxygenation (ECMO) training simulation, and said that much could be learned from the Fundamentals of Laparoscopic Surgery program, a simulation program required in order to get certification and graduate in a general surgery residency. The system includes a kit that allows surgeons to practice at home and online to learn skills, he said.
"We need to build ‘Fundamentals of Endovascular Surgery,’ " he asserted. "We can do this and do it relatively easily and relatively cheaply. ... It’s not debatable as to whether this is of value. It’s debatable whether we can figure out how to do it and institute it into vascular surgery on a daily basis."
A Global Concern
Just what level of simulation is appropriate is an international question, and a recent analysis of how best to expand the use of simulation in open vascular surgical training was presented by Dr. Vikas A. Pandey and Dr. John H.N. Wolfe of the Imperial College Healthcare NHS Trust, London. In a recent article, they discussed one major issue in simulation that has created considerable concern in the vascular community especially – the comparative ease of simulating endovascular and laparoscopic approaches (which can be simulated using a two-dimensional imaging approach, and is one of the driving forces behind the simulation movement) as compared with open surgery, which requires a three-dimensional field to be representative of the procedure and is much more difficult to simulate effectively (J. Vasc. Surg. 2012 [doi: 10.1016/j.jvs.2012.04.015]).
Dr. Pandey and Dr. Wolfe indicated that a wide range of simulators exist for all aspects of vascular surgical training, and that these vary in complexity and price, from simulators suitable for use at home or in a local skills laboratory to those that can only be practically implemented at a regional skills center.
But even the latter are not the highest levels of simulation required. The authors suggested that higher levels of simulation training require more stringent considerations. "Where surgical procedures are not commonly performed or expertise is required for a new innovation, it is more appropriate to have national or internationally based workshops under the auspices of surgical boards or societies," they wrote.
Finding consensus on the best method for implementing simulation-based training in a surgical curriculum also remains a thorny subject. Dr. Zevin and his colleagues from the United States reported on their attempt to achieve international consensus. They used an iterative, online Delphi survey to develop a consensus among 24 international experts with training ranging from general surgery to vascular surgery. The final framework agreed upon for resident training was the need for predevelopment analysis of trainees; cognitive, psychomotor, and team-based simulation training; and methods of curriculum validation, evaluation, and improvement. A consensus was also reached by greater than 80% on the need for simulation for maintenance of training.
But Does It Work?
Dr. Lumsden’s position, and that of all strong proponents of simulation, was called into question in the Vascular Annual Meeting debate by Dr. Makaroun of the University of Pittsburgh Medical Center, who stated that it is a waste of time and money.
"Simulation makes sense. ... It is safe and can’t hurt patients, it should improve education and learning, and it should improve clinical performance; it should help in the evaluation of competency. ... It has already been adopted by everybody ... and everywhere I look, simulation is there," he acknowledged. However, he said, "it is crucial that we recognize that the success of the flight simulator [the example always touted as a key triumph for simulation] does not translate well in simulating everything – in particular, not in highly complex, highly indeterminate situations such as human biology and behavior."
For effective simulation, he stated, you need an effective teacher, you need repetition, you need to measure the performance and get feedback, and thus you need the accessibility of local simulation facilities – meaning hundreds, if not thousands, of simulators nationwide.
Major simulation centers have cost between $20 million and $40 million to build, and the simulators in those facilities can cost in the hundreds of thousands of dollars, he added. Perhaps the priciest commodity of all is the time of a faculty trainer, he suggested.
Dr. Makaroun complained that industry is actively promoting this entire area and that the ultimate price tag is in the billions. So the case against simulation is that it is too expensive.
"So far, there is absolutely no data to indicate that it has improved the surgical skills of graduates who trained on simulators vs. those who did not," said Dr. Makaroun. There are also "absolutely no data" indicating a link between simulator use and improved patient outcomes and safety.
In fact, he said, most simulator studies being reported in the literature simply show that somebody who practiced a task on a simulator was better at doing that same task on the simulator after practice than before. "Are we really surprised that vascular residents can learn something after 2 days?" he asked. Studies are needed to assess whether surgeons trained on simulators performed actual procedures better at 6 months after training than did those who had received standard training.
Some general surgery residents also are still conflicted about the value of simulation training. In the in-depth survey cited above, 25 general surgery residents, all of whom were exposed to simulation training at the Texas A&M Health Science Center College of Medicine, were almost equally divided as to whether "ACGME should require a simulation curriculum in surgery residency" (52.1%, yes; 47.8%, no). Further questioning elicited concerns about whether there was any evidence of efficacy of simulation in surgical residency training beyond the traditional approach, and how simulation could not replace "real experience" on patients.
So, despite the growing consensus that simulation has a role in surgical education and training, there is less consensus as to how to evaluate the effectiveness (if any) of such training when the rubber (or plastic model) meets the operating room.
And if effectiveness cannot be proven, how can the tremendous investments in technology, time, and facilities be justified?
What Next?
In the end, the problems of cost, level, and effectiveness of simulation remain to be resolved. "Unfortunately, the promise of patient-specific, high-fidelity, virtual reality vascular surgical simulation remains largely unfulfilled due to the enormous development costs and the computational complexity associated with mimicking the response of tissue to deformation," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery (J. Vasc. Surg. 2012;55:1801-9).
He added that "low-fidelity, low-cost simulation is effective for teaching basic surgical skills such as suturing or knot-tying, or the sequence of steps in an operation to novice surgeons, but is remarkably ineffective for advanced learners where fidelity is critical."
Dainty morsels may apply for early trainees, but are pig-in-a-python meals required for the more advanced?
The lines are drawn, with seemingly the majority of educators, government regulators, and the general public convinced that the logic of simulators is undeniable, and that enough data from fields outside of surgery exist to justify the wide adoption of simulators in surgical training.
Given the trajectory of modern medicine, it seems that more rather than less technology is likely to be the answer – or at least the answer most acceptable to device-driven societies such as the United States and Western Europe, where these approaches are being pioneered. Hence, the continued use and expansion of simulation in surgical training seem inevitable.
Dr. Lumsden reported that he had no conflicts of interest other than working at the DeBakey Institute, where simulation training was of great importance. Dr. Zevin, Dr. Eidt, Dr. Makaroun, and Dr. Pandey reported no relevant disclosures. Dr. Wolfe reported receiving funding from Limbs & Things.
Simulation is an increasing part of surgical training and a critical component in producing the next generation of surgeons – but it is also the focus of questions and controversy, as evidenced by a spate of recent journal articles and by views expressed at a special session of the 2012 Vascular Annual Meeting.
Reasons for the interest in simulation include duty hour restrictions, more complex and automated procedures, and stricter quality assurance programs such as pay for performance – all of which mandate the need to practice and perfect difficult techniques. The push toward simulation is projected to affect not only residents in early training but also established surgeons pursuing Maintenance of Certification (MOC).
Questions seem to have moved beyond simply whether simulation is needed to how much simulation training is needed and how to pay for it. Can simulation training be administered as dainty morsels nibbled over time, or does it need to be swallowed all at once – the pig-in-a-python approach?
It all depends on what level of simulation is necessary. Suturing can be practiced on rubber tubing or on the latest synthetic bioskin products, which mimic multiple skin layers. Arterial procedures can be mimicked by operating on tubing in plastic see-through dummies, or by using robotic simulators to operate on virtual patients generated as three-dimensional CT scans. And the standard of performing simulated operations in the cadaver lab still exists.
Everything ranging from relatively simple, individual technique simulators that cost comparatively little to massive, regional simulation centers that cost hundreds of thousands of dollars are now being used in surgical training. Highly visible examples of what is possible (if you can afford it) are the Goodman Simulation Center at Stanford (Calif.) University, the Stony Brook Medicine Surgical Skills Center at the State University of New York at Stony Brook, the Methodist DeBakey Heart and Vascular Center in Houston, and the University of South Florida Center for Advanced Medical Learning and Simulation in Tampa.
Since 2008, the Accreditation Council for Graduate Medical Education (ACGME) has required, for general surgical training, that resources "include simulation and skills laboratories. These facilities must address acquisition and maintenance of skills with a competency-based method of evaluation." But the extent to which simulation should play a role in training, how much simulation should be required, and how it should be evaluated are subject to debate. The only consensus seems to be that something is needed, and simulation is here to stay, like it or not.
Why Now?
The need for simulation training appears to be driven by several intersecting forces – social and economic problems for which simulation claims to be the solution.
"During the past 100 years, time-based apprentice-type surgical training programs have produced many superb surgeons. However, with restrictions in residency work hours, increasing emphasis on patient safety, and rising costs for training in the operating room, trainees now have fewer opportunities to train in the operating room," according to Dr. Boris Zevin of the University of Toronto and his colleagues.
"The major driving force behind the call to incorporate simulation into surgical training comes from the need to improve patient safety and to shorten the learning curve in the operating room," Dr. Zevin, leader of an international survey that assessed simulation-based training in surgery, and his colleagues wrote in the Journal of the American College of Surgeons (2012 [doi: 10.1016/j.jamcollsurg.2012.05.035]).
In a recent survey, residents in a single surgical simulation training program expressed concern that the need for simulation wasn’t being driven by the health care system or from within the surgical community. Two candid opinions in the survey stated that simulation is "filling this public thirst for there being a uniform, regulated – on a national level – program by which we are all practicing on, basically, objects, before practicing on people," and "a core component of just some way for the health care industry to prove that there are competent people and this is what they are competent in" (Surgery 2012;151:815-21).
Ultimately, one of the strongest pressures to alter resident education may well be financial. According to Dr. John F. Eidt of the University of Arkansas for Medical Sciences, Little Rock, a leading voice for simulation training in vascular surgery education, "The federal government is the primary source of funding for graduate medical education (GME) through supplementation of hospital reimbursement. Recent events threaten to drastically alter GME funding. ... The Medicare Payment Advisory Commission has stated that up to 50% of indirect GME reimbursement is not ‘empirically justified’ by actual hospital costs associated with education," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery.
At the Vascular Annual Meeting, he reiterated his support for simulator training, and discussed the success of the aviation model of simulation in detail.
The Case for Simulation
If adopting simulation is all but inevitable, how best to do it? The dainty morsels vs. pig-in-a-python approach was at the crux of a debate held at the Vascular Annual Meeting session. On the pro-simulation side, Dr. Alan B. Lumsden of the Methodist DeBakey Heart and Vascular Center, Houston, saw simulation as "the wave of the future."
Dr. Lumsden was adamant in saying that the costly, high-fidelity virtual reality systems are not what simulation training for residents should be about. He used CPR dummies as an example of one of the most effective simulators. These cost "about a hundred bucks a pop," compared with some $250,000 systems, he noted, adding that almost every senior vascular surgeon beside him on the podium learned how to do endovascular procedures on foam rubber iliac and aortic stenosis models (including his debate adversary, Dr. Michael S. Makaroun).
"The question is not whether simulation is valid, but the question is whether we as vascular surgeons can figure out how to take what we have learned and learn how to apply that [to simulations]." His major point was that there should be a focus on simulation and how to standardize its use. He indicated that in single tasks, simulation could be made ideal, such that eventually "we should be all doing the procedure in the exact same way," and he gave the example of groin puncture to access a femoral vessel as one good place to start.
Dr. Lumsden also pointed out the critical validity of simulation for team training. He used the example of extracorporeal membrane oxygenation (ECMO) training simulation, and said that much could be learned from the Fundamentals of Laparoscopic Surgery program, a simulation program required in order to get certification and graduate in a general surgery residency. The system includes a kit that allows surgeons to practice at home and online to learn skills, he said.
"We need to build ‘Fundamentals of Endovascular Surgery,’ " he asserted. "We can do this and do it relatively easily and relatively cheaply. ... It’s not debatable as to whether this is of value. It’s debatable whether we can figure out how to do it and institute it into vascular surgery on a daily basis."
A Global Concern
Just what level of simulation is appropriate is an international question, and a recent analysis of how best to expand the use of simulation in open vascular surgical training was presented by Dr. Vikas A. Pandey and Dr. John H.N. Wolfe of the Imperial College Healthcare NHS Trust, London. In a recent article, they discussed one major issue in simulation that has created considerable concern in the vascular community especially – the comparative ease of simulating endovascular and laparoscopic approaches (which can be simulated using a two-dimensional imaging approach, and is one of the driving forces behind the simulation movement) as compared with open surgery, which requires a three-dimensional field to be representative of the procedure and is much more difficult to simulate effectively (J. Vasc. Surg. 2012 [doi: 10.1016/j.jvs.2012.04.015]).
Dr. Pandey and Dr. Wolfe indicated that a wide range of simulators exist for all aspects of vascular surgical training, and that these vary in complexity and price, from simulators suitable for use at home or in a local skills laboratory to those that can only be practically implemented at a regional skills center.
But even the latter are not the highest levels of simulation required. The authors suggested that higher levels of simulation training require more stringent considerations. "Where surgical procedures are not commonly performed or expertise is required for a new innovation, it is more appropriate to have national or internationally based workshops under the auspices of surgical boards or societies," they wrote.
Finding consensus on the best method for implementing simulation-based training in a surgical curriculum also remains a thorny subject. Dr. Zevin and his colleagues from the United States reported on their attempt to achieve international consensus. They used an iterative, online Delphi survey to develop a consensus among 24 international experts with training ranging from general surgery to vascular surgery. The final framework agreed upon for resident training was the need for predevelopment analysis of trainees; cognitive, psychomotor, and team-based simulation training; and methods of curriculum validation, evaluation, and improvement. A consensus was also reached by greater than 80% on the need for simulation for maintenance of training.
But Does It Work?
Dr. Lumsden’s position, and that of all strong proponents of simulation, was called into question in the Vascular Annual Meeting debate by Dr. Makaroun of the University of Pittsburgh Medical Center, who stated that it is a waste of time and money.
"Simulation makes sense. ... It is safe and can’t hurt patients, it should improve education and learning, and it should improve clinical performance; it should help in the evaluation of competency. ... It has already been adopted by everybody ... and everywhere I look, simulation is there," he acknowledged. However, he said, "it is crucial that we recognize that the success of the flight simulator [the example always touted as a key triumph for simulation] does not translate well in simulating everything – in particular, not in highly complex, highly indeterminate situations such as human biology and behavior."
For effective simulation, he stated, you need an effective teacher, you need repetition, you need to measure the performance and get feedback, and thus you need the accessibility of local simulation facilities – meaning hundreds, if not thousands, of simulators nationwide.
Major simulation centers have cost between $20 million and $40 million to build, and the simulators in those facilities can cost in the hundreds of thousands of dollars, he added. Perhaps the priciest commodity of all is the time of a faculty trainer, he suggested.
Dr. Makaroun complained that industry is actively promoting this entire area and that the ultimate price tag is in the billions. So the case against simulation is that it is too expensive.
"So far, there is absolutely no data to indicate that it has improved the surgical skills of graduates who trained on simulators vs. those who did not," said Dr. Makaroun. There are also "absolutely no data" indicating a link between simulator use and improved patient outcomes and safety.
In fact, he said, most simulator studies being reported in the literature simply show that somebody who practiced a task on a simulator was better at doing that same task on the simulator after practice than before. "Are we really surprised that vascular residents can learn something after 2 days?" he asked. Studies are needed to assess whether surgeons trained on simulators performed actual procedures better at 6 months after training than did those who had received standard training.
Some general surgery residents also are still conflicted about the value of simulation training. In the in-depth survey cited above, 25 general surgery residents, all of whom were exposed to simulation training at the Texas A&M Health Science Center College of Medicine, were almost equally divided as to whether "ACGME should require a simulation curriculum in surgery residency" (52.1%, yes; 47.8%, no). Further questioning elicited concerns about whether there was any evidence of efficacy of simulation in surgical residency training beyond the traditional approach, and how simulation could not replace "real experience" on patients.
So, despite the growing consensus that simulation has a role in surgical education and training, there is less consensus as to how to evaluate the effectiveness (if any) of such training when the rubber (or plastic model) meets the operating room.
And if effectiveness cannot be proven, how can the tremendous investments in technology, time, and facilities be justified?
What Next?
In the end, the problems of cost, level, and effectiveness of simulation remain to be resolved. "Unfortunately, the promise of patient-specific, high-fidelity, virtual reality vascular surgical simulation remains largely unfulfilled due to the enormous development costs and the computational complexity associated with mimicking the response of tissue to deformation," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery (J. Vasc. Surg. 2012;55:1801-9).
He added that "low-fidelity, low-cost simulation is effective for teaching basic surgical skills such as suturing or knot-tying, or the sequence of steps in an operation to novice surgeons, but is remarkably ineffective for advanced learners where fidelity is critical."
Dainty morsels may apply for early trainees, but are pig-in-a-python meals required for the more advanced?
The lines are drawn, with seemingly the majority of educators, government regulators, and the general public convinced that the logic of simulators is undeniable, and that enough data from fields outside of surgery exist to justify the wide adoption of simulators in surgical training.
Given the trajectory of modern medicine, it seems that more rather than less technology is likely to be the answer – or at least the answer most acceptable to device-driven societies such as the United States and Western Europe, where these approaches are being pioneered. Hence, the continued use and expansion of simulation in surgical training seem inevitable.
Dr. Lumsden reported that he had no conflicts of interest other than working at the DeBakey Institute, where simulation training was of great importance. Dr. Zevin, Dr. Eidt, Dr. Makaroun, and Dr. Pandey reported no relevant disclosures. Dr. Wolfe reported receiving funding from Limbs & Things.
Simulation is an increasing part of surgical training and a critical component in producing the next generation of surgeons – but it is also the focus of questions and controversy, as evidenced by a spate of recent journal articles and by views expressed at a special session of the 2012 Vascular Annual Meeting.
Reasons for the interest in simulation include duty hour restrictions, more complex and automated procedures, and stricter quality assurance programs such as pay for performance – all of which mandate the need to practice and perfect difficult techniques. The push toward simulation is projected to affect not only residents in early training but also established surgeons pursuing Maintenance of Certification (MOC).
Questions seem to have moved beyond simply whether simulation is needed to how much simulation training is needed and how to pay for it. Can simulation training be administered as dainty morsels nibbled over time, or does it need to be swallowed all at once – the pig-in-a-python approach?
It all depends on what level of simulation is necessary. Suturing can be practiced on rubber tubing or on the latest synthetic bioskin products, which mimic multiple skin layers. Arterial procedures can be mimicked by operating on tubing in plastic see-through dummies, or by using robotic simulators to operate on virtual patients generated as three-dimensional CT scans. And the standard of performing simulated operations in the cadaver lab still exists.
Everything ranging from relatively simple, individual technique simulators that cost comparatively little to massive, regional simulation centers that cost hundreds of thousands of dollars are now being used in surgical training. Highly visible examples of what is possible (if you can afford it) are the Goodman Simulation Center at Stanford (Calif.) University, the Stony Brook Medicine Surgical Skills Center at the State University of New York at Stony Brook, the Methodist DeBakey Heart and Vascular Center in Houston, and the University of South Florida Center for Advanced Medical Learning and Simulation in Tampa.
Since 2008, the Accreditation Council for Graduate Medical Education (ACGME) has required, for general surgical training, that resources "include simulation and skills laboratories. These facilities must address acquisition and maintenance of skills with a competency-based method of evaluation." But the extent to which simulation should play a role in training, how much simulation should be required, and how it should be evaluated are subject to debate. The only consensus seems to be that something is needed, and simulation is here to stay, like it or not.
Why Now?
The need for simulation training appears to be driven by several intersecting forces – social and economic problems for which simulation claims to be the solution.
"During the past 100 years, time-based apprentice-type surgical training programs have produced many superb surgeons. However, with restrictions in residency work hours, increasing emphasis on patient safety, and rising costs for training in the operating room, trainees now have fewer opportunities to train in the operating room," according to Dr. Boris Zevin of the University of Toronto and his colleagues.
"The major driving force behind the call to incorporate simulation into surgical training comes from the need to improve patient safety and to shorten the learning curve in the operating room," Dr. Zevin, leader of an international survey that assessed simulation-based training in surgery, and his colleagues wrote in the Journal of the American College of Surgeons (2012 [doi: 10.1016/j.jamcollsurg.2012.05.035]).
In a recent survey, residents in a single surgical simulation training program expressed concern that the need for simulation wasn’t being driven by the health care system or from within the surgical community. Two candid opinions in the survey stated that simulation is "filling this public thirst for there being a uniform, regulated – on a national level – program by which we are all practicing on, basically, objects, before practicing on people," and "a core component of just some way for the health care industry to prove that there are competent people and this is what they are competent in" (Surgery 2012;151:815-21).
Ultimately, one of the strongest pressures to alter resident education may well be financial. According to Dr. John F. Eidt of the University of Arkansas for Medical Sciences, Little Rock, a leading voice for simulation training in vascular surgery education, "The federal government is the primary source of funding for graduate medical education (GME) through supplementation of hospital reimbursement. Recent events threaten to drastically alter GME funding. ... The Medicare Payment Advisory Commission has stated that up to 50% of indirect GME reimbursement is not ‘empirically justified’ by actual hospital costs associated with education," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery.
At the Vascular Annual Meeting, he reiterated his support for simulator training, and discussed the success of the aviation model of simulation in detail.
The Case for Simulation
If adopting simulation is all but inevitable, how best to do it? The dainty morsels vs. pig-in-a-python approach was at the crux of a debate held at the Vascular Annual Meeting session. On the pro-simulation side, Dr. Alan B. Lumsden of the Methodist DeBakey Heart and Vascular Center, Houston, saw simulation as "the wave of the future."
Dr. Lumsden was adamant in saying that the costly, high-fidelity virtual reality systems are not what simulation training for residents should be about. He used CPR dummies as an example of one of the most effective simulators. These cost "about a hundred bucks a pop," compared with some $250,000 systems, he noted, adding that almost every senior vascular surgeon beside him on the podium learned how to do endovascular procedures on foam rubber iliac and aortic stenosis models (including his debate adversary, Dr. Michael S. Makaroun).
"The question is not whether simulation is valid, but the question is whether we as vascular surgeons can figure out how to take what we have learned and learn how to apply that [to simulations]." His major point was that there should be a focus on simulation and how to standardize its use. He indicated that in single tasks, simulation could be made ideal, such that eventually "we should be all doing the procedure in the exact same way," and he gave the example of groin puncture to access a femoral vessel as one good place to start.
Dr. Lumsden also pointed out the critical validity of simulation for team training. He used the example of extracorporeal membrane oxygenation (ECMO) training simulation, and said that much could be learned from the Fundamentals of Laparoscopic Surgery program, a simulation program required in order to get certification and graduate in a general surgery residency. The system includes a kit that allows surgeons to practice at home and online to learn skills, he said.
"We need to build ‘Fundamentals of Endovascular Surgery,’ " he asserted. "We can do this and do it relatively easily and relatively cheaply. ... It’s not debatable as to whether this is of value. It’s debatable whether we can figure out how to do it and institute it into vascular surgery on a daily basis."
A Global Concern
Just what level of simulation is appropriate is an international question, and a recent analysis of how best to expand the use of simulation in open vascular surgical training was presented by Dr. Vikas A. Pandey and Dr. John H.N. Wolfe of the Imperial College Healthcare NHS Trust, London. In a recent article, they discussed one major issue in simulation that has created considerable concern in the vascular community especially – the comparative ease of simulating endovascular and laparoscopic approaches (which can be simulated using a two-dimensional imaging approach, and is one of the driving forces behind the simulation movement) as compared with open surgery, which requires a three-dimensional field to be representative of the procedure and is much more difficult to simulate effectively (J. Vasc. Surg. 2012 [doi: 10.1016/j.jvs.2012.04.015]).
Dr. Pandey and Dr. Wolfe indicated that a wide range of simulators exist for all aspects of vascular surgical training, and that these vary in complexity and price, from simulators suitable for use at home or in a local skills laboratory to those that can only be practically implemented at a regional skills center.
But even the latter are not the highest levels of simulation required. The authors suggested that higher levels of simulation training require more stringent considerations. "Where surgical procedures are not commonly performed or expertise is required for a new innovation, it is more appropriate to have national or internationally based workshops under the auspices of surgical boards or societies," they wrote.
Finding consensus on the best method for implementing simulation-based training in a surgical curriculum also remains a thorny subject. Dr. Zevin and his colleagues from the United States reported on their attempt to achieve international consensus. They used an iterative, online Delphi survey to develop a consensus among 24 international experts with training ranging from general surgery to vascular surgery. The final framework agreed upon for resident training was the need for predevelopment analysis of trainees; cognitive, psychomotor, and team-based simulation training; and methods of curriculum validation, evaluation, and improvement. A consensus was also reached by greater than 80% on the need for simulation for maintenance of training.
But Does It Work?
Dr. Lumsden’s position, and that of all strong proponents of simulation, was called into question in the Vascular Annual Meeting debate by Dr. Makaroun of the University of Pittsburgh Medical Center, who stated that it is a waste of time and money.
"Simulation makes sense. ... It is safe and can’t hurt patients, it should improve education and learning, and it should improve clinical performance; it should help in the evaluation of competency. ... It has already been adopted by everybody ... and everywhere I look, simulation is there," he acknowledged. However, he said, "it is crucial that we recognize that the success of the flight simulator [the example always touted as a key triumph for simulation] does not translate well in simulating everything – in particular, not in highly complex, highly indeterminate situations such as human biology and behavior."
For effective simulation, he stated, you need an effective teacher, you need repetition, you need to measure the performance and get feedback, and thus you need the accessibility of local simulation facilities – meaning hundreds, if not thousands, of simulators nationwide.
Major simulation centers have cost between $20 million and $40 million to build, and the simulators in those facilities can cost in the hundreds of thousands of dollars, he added. Perhaps the priciest commodity of all is the time of a faculty trainer, he suggested.
Dr. Makaroun complained that industry is actively promoting this entire area and that the ultimate price tag is in the billions. So the case against simulation is that it is too expensive.
"So far, there is absolutely no data to indicate that it has improved the surgical skills of graduates who trained on simulators vs. those who did not," said Dr. Makaroun. There are also "absolutely no data" indicating a link between simulator use and improved patient outcomes and safety.
In fact, he said, most simulator studies being reported in the literature simply show that somebody who practiced a task on a simulator was better at doing that same task on the simulator after practice than before. "Are we really surprised that vascular residents can learn something after 2 days?" he asked. Studies are needed to assess whether surgeons trained on simulators performed actual procedures better at 6 months after training than did those who had received standard training.
Some general surgery residents also are still conflicted about the value of simulation training. In the in-depth survey cited above, 25 general surgery residents, all of whom were exposed to simulation training at the Texas A&M Health Science Center College of Medicine, were almost equally divided as to whether "ACGME should require a simulation curriculum in surgery residency" (52.1%, yes; 47.8%, no). Further questioning elicited concerns about whether there was any evidence of efficacy of simulation in surgical residency training beyond the traditional approach, and how simulation could not replace "real experience" on patients.
So, despite the growing consensus that simulation has a role in surgical education and training, there is less consensus as to how to evaluate the effectiveness (if any) of such training when the rubber (or plastic model) meets the operating room.
And if effectiveness cannot be proven, how can the tremendous investments in technology, time, and facilities be justified?
What Next?
In the end, the problems of cost, level, and effectiveness of simulation remain to be resolved. "Unfortunately, the promise of patient-specific, high-fidelity, virtual reality vascular surgical simulation remains largely unfulfilled due to the enormous development costs and the computational complexity associated with mimicking the response of tissue to deformation," Dr. Eidt said during his presidential address at the annual meeting of the Southern Association for Vascular Surgery (J. Vasc. Surg. 2012;55:1801-9).
He added that "low-fidelity, low-cost simulation is effective for teaching basic surgical skills such as suturing or knot-tying, or the sequence of steps in an operation to novice surgeons, but is remarkably ineffective for advanced learners where fidelity is critical."
Dainty morsels may apply for early trainees, but are pig-in-a-python meals required for the more advanced?
The lines are drawn, with seemingly the majority of educators, government regulators, and the general public convinced that the logic of simulators is undeniable, and that enough data from fields outside of surgery exist to justify the wide adoption of simulators in surgical training.
Given the trajectory of modern medicine, it seems that more rather than less technology is likely to be the answer – or at least the answer most acceptable to device-driven societies such as the United States and Western Europe, where these approaches are being pioneered. Hence, the continued use and expansion of simulation in surgical training seem inevitable.
Dr. Lumsden reported that he had no conflicts of interest other than working at the DeBakey Institute, where simulation training was of great importance. Dr. Zevin, Dr. Eidt, Dr. Makaroun, and Dr. Pandey reported no relevant disclosures. Dr. Wolfe reported receiving funding from Limbs & Things.
AAA Rescreening Worthwhile in Dollars Per Life
A new model demonstrated that at least one additional screening for abdominal aortic aneurysm was more cost effective in dollars per quality-adjusted life-year than was the single screening currently mandated for select populations in countries like the United States and England.
Previous decision models agreed that the optimal AAA screening protocol is a once-in-a-lifetime scan that is performed in men at age 65. However, none of these models examined the additional benefit of rescreening individuals whose aortic diameters approached but did not exceed the defined aneurysm threshold.
Dr. Rikke Søgaard and his colleagues developed their new model to determine if there was an optimal rescreening schedule for patients. They tested four screening strategies (no screening, once per lifetime screening, twice per lifetime screening with a 5-year interval, and lifetime screening every 5 years) for a hypothetical cohort of 100,000 men aged 65 years who were taken from the general population. Each individual was assigned to one of the four strategies. The researchers then compared the estimated lifetime costs and benefits of the four AAA strategies for this population.
The model used microsimulation of 6-month cycles to provide detailed epidemiologic results for each of the strategies, based on key events (detection, symptoms, rupture, and death) during a simulated lifetime. Apart from rupture rates taken from a systematic review, parameter estimates were the result of original analyses composed of a combination of research registries from two Danish screening trials, the Danish Vascular Registry, and national registries for causes of death.
Assuming a 12% per year incidental detection rate of aneurysms measuring 55 mm and larger, the model predicted that 2,469 men would be detected with a clinically relevant aneurysm. A single rescreening after 5 years of individuals without initial aneurysms who had an initial aortic diameter of 25-29 mm would detect an additional 452 men per 100,000 of those originally screened, whereas lifetime rescreening every 5 years thereafter would detect a total of 794 men with a clinically relevant aneurysm per 100,000.
Most of these aneurysms were smaller than the threshold for surgery, but appropriate for watchful waiting with rescreening, according to Dr. Søgaard of the University of Southern Denmark, Odense, and his colleagues.
The researchers found that elective surgeries would increase from 861 to 1,390 with a once-per-lifetime screening, to 1,496 for a single rescreening after 5 years, and to 1,530 with rescreening every 5 years for life. The rate of aneurysm-related mortality dropped with initial screening from 788 to 538 per 100,000, further falling to 520 and 511 for the single rescreening and the lifetime rescreening, respectively. "This decrease was the result of replacing acute surgery with elective surgery," the researchers stated (BMJ 2012 July 5 [doi:10.1136/bmj.e4276]).
The cost effectiveness of rescreening had not previously been studied, according to the authors. They determined that according to their model, there was a 92% probability that any rescreening protocol would be cost effective if it were at or below a threshold of £20,000 (24,790 euros and $31,460) per quality-adjusted life-year. They estimated that the incremental cost effectiveness was £10,013 per QALY, well under the threshold.
However, Dr. Søgaard and his colleagues also pointed out that substantial uncertainty surrounded this ratio, and "with an average incremental cost-effectiveness ratio of lifetime rescreening of £29,680, the optimal screening strategy is indeterminate"
"This study has policy relevance for two different scenarios. In Denmark, where no national guidance has been issued, it suggests that screening will be cost effective. In England and Scotland, where screening is currently being implemented (as is also the case in the United States), this study supports further consideration of rescreening, at least once," the researchers concluded.
The study was funded by the Health Research Fund of Central Denmark Region and the Research Fund of Viborg Hospital. The researchers disclosed financial support from these two agencies but no other relevant disclosures.
A new model demonstrated that at least one additional screening for abdominal aortic aneurysm was more cost effective in dollars per quality-adjusted life-year than was the single screening currently mandated for select populations in countries like the United States and England.
Previous decision models agreed that the optimal AAA screening protocol is a once-in-a-lifetime scan that is performed in men at age 65. However, none of these models examined the additional benefit of rescreening individuals whose aortic diameters approached but did not exceed the defined aneurysm threshold.
Dr. Rikke Søgaard and his colleagues developed their new model to determine if there was an optimal rescreening schedule for patients. They tested four screening strategies (no screening, once per lifetime screening, twice per lifetime screening with a 5-year interval, and lifetime screening every 5 years) for a hypothetical cohort of 100,000 men aged 65 years who were taken from the general population. Each individual was assigned to one of the four strategies. The researchers then compared the estimated lifetime costs and benefits of the four AAA strategies for this population.
The model used microsimulation of 6-month cycles to provide detailed epidemiologic results for each of the strategies, based on key events (detection, symptoms, rupture, and death) during a simulated lifetime. Apart from rupture rates taken from a systematic review, parameter estimates were the result of original analyses composed of a combination of research registries from two Danish screening trials, the Danish Vascular Registry, and national registries for causes of death.
Assuming a 12% per year incidental detection rate of aneurysms measuring 55 mm and larger, the model predicted that 2,469 men would be detected with a clinically relevant aneurysm. A single rescreening after 5 years of individuals without initial aneurysms who had an initial aortic diameter of 25-29 mm would detect an additional 452 men per 100,000 of those originally screened, whereas lifetime rescreening every 5 years thereafter would detect a total of 794 men with a clinically relevant aneurysm per 100,000.
Most of these aneurysms were smaller than the threshold for surgery, but appropriate for watchful waiting with rescreening, according to Dr. Søgaard of the University of Southern Denmark, Odense, and his colleagues.
The researchers found that elective surgeries would increase from 861 to 1,390 with a once-per-lifetime screening, to 1,496 for a single rescreening after 5 years, and to 1,530 with rescreening every 5 years for life. The rate of aneurysm-related mortality dropped with initial screening from 788 to 538 per 100,000, further falling to 520 and 511 for the single rescreening and the lifetime rescreening, respectively. "This decrease was the result of replacing acute surgery with elective surgery," the researchers stated (BMJ 2012 July 5 [doi:10.1136/bmj.e4276]).
The cost effectiveness of rescreening had not previously been studied, according to the authors. They determined that according to their model, there was a 92% probability that any rescreening protocol would be cost effective if it were at or below a threshold of £20,000 (24,790 euros and $31,460) per quality-adjusted life-year. They estimated that the incremental cost effectiveness was £10,013 per QALY, well under the threshold.
However, Dr. Søgaard and his colleagues also pointed out that substantial uncertainty surrounded this ratio, and "with an average incremental cost-effectiveness ratio of lifetime rescreening of £29,680, the optimal screening strategy is indeterminate"
"This study has policy relevance for two different scenarios. In Denmark, where no national guidance has been issued, it suggests that screening will be cost effective. In England and Scotland, where screening is currently being implemented (as is also the case in the United States), this study supports further consideration of rescreening, at least once," the researchers concluded.
The study was funded by the Health Research Fund of Central Denmark Region and the Research Fund of Viborg Hospital. The researchers disclosed financial support from these two agencies but no other relevant disclosures.
A new model demonstrated that at least one additional screening for abdominal aortic aneurysm was more cost effective in dollars per quality-adjusted life-year than was the single screening currently mandated for select populations in countries like the United States and England.
Previous decision models agreed that the optimal AAA screening protocol is a once-in-a-lifetime scan that is performed in men at age 65. However, none of these models examined the additional benefit of rescreening individuals whose aortic diameters approached but did not exceed the defined aneurysm threshold.
Dr. Rikke Søgaard and his colleagues developed their new model to determine if there was an optimal rescreening schedule for patients. They tested four screening strategies (no screening, once per lifetime screening, twice per lifetime screening with a 5-year interval, and lifetime screening every 5 years) for a hypothetical cohort of 100,000 men aged 65 years who were taken from the general population. Each individual was assigned to one of the four strategies. The researchers then compared the estimated lifetime costs and benefits of the four AAA strategies for this population.
The model used microsimulation of 6-month cycles to provide detailed epidemiologic results for each of the strategies, based on key events (detection, symptoms, rupture, and death) during a simulated lifetime. Apart from rupture rates taken from a systematic review, parameter estimates were the result of original analyses composed of a combination of research registries from two Danish screening trials, the Danish Vascular Registry, and national registries for causes of death.
Assuming a 12% per year incidental detection rate of aneurysms measuring 55 mm and larger, the model predicted that 2,469 men would be detected with a clinically relevant aneurysm. A single rescreening after 5 years of individuals without initial aneurysms who had an initial aortic diameter of 25-29 mm would detect an additional 452 men per 100,000 of those originally screened, whereas lifetime rescreening every 5 years thereafter would detect a total of 794 men with a clinically relevant aneurysm per 100,000.
Most of these aneurysms were smaller than the threshold for surgery, but appropriate for watchful waiting with rescreening, according to Dr. Søgaard of the University of Southern Denmark, Odense, and his colleagues.
The researchers found that elective surgeries would increase from 861 to 1,390 with a once-per-lifetime screening, to 1,496 for a single rescreening after 5 years, and to 1,530 with rescreening every 5 years for life. The rate of aneurysm-related mortality dropped with initial screening from 788 to 538 per 100,000, further falling to 520 and 511 for the single rescreening and the lifetime rescreening, respectively. "This decrease was the result of replacing acute surgery with elective surgery," the researchers stated (BMJ 2012 July 5 [doi:10.1136/bmj.e4276]).
The cost effectiveness of rescreening had not previously been studied, according to the authors. They determined that according to their model, there was a 92% probability that any rescreening protocol would be cost effective if it were at or below a threshold of £20,000 (24,790 euros and $31,460) per quality-adjusted life-year. They estimated that the incremental cost effectiveness was £10,013 per QALY, well under the threshold.
However, Dr. Søgaard and his colleagues also pointed out that substantial uncertainty surrounded this ratio, and "with an average incremental cost-effectiveness ratio of lifetime rescreening of £29,680, the optimal screening strategy is indeterminate"
"This study has policy relevance for two different scenarios. In Denmark, where no national guidance has been issued, it suggests that screening will be cost effective. In England and Scotland, where screening is currently being implemented (as is also the case in the United States), this study supports further consideration of rescreening, at least once," the researchers concluded.
The study was funded by the Health Research Fund of Central Denmark Region and the Research Fund of Viborg Hospital. The researchers disclosed financial support from these two agencies but no other relevant disclosures.
FROM BMJ
Major Finding: For men with an initial aortic diameter of 25-29 mm, a single rescreening after 5 years would benefit 452 per 100,000 men. Lifetime screening every 5 years would benefit 794 per 100,000, but at a nearly threefold higher cost per QALY.
Data Source: Researchers used a decision analytical model to assess a hypothetical cohort of 65-year-old men from the general population, including ad hoc parameter estimates from the Danish Vascular Registry and other registries.
Disclosures: The study was funded by the Health Research Fund of Central Denmark Region and the Research Fund of Viborg Hospital. The researchers disclosed financial support from these two agencies, but no other relevant disclosures.
Rudolph Matas, M.D.
Rudolph Matas, M.D, is yet another of the famous surgeons of the past 2 centuries to whom the sobriquet "father of Vascular Surgery" has been applied, primarily for his pioneering studies on and surgical treatment of aneurysms before the era of prosthetic grafts. This September is the 50th anniversary of his death.
His amazingly long career (he was born in 1860 on a Louisiana plantation and lived to age 97) spanned the period in which belief in spontaneous generation and contaminated airs and humors transformed into a new germ theory of disease. He saw the revolutions begun by Pasteur, Koch, and Lister, the development of sterilization techniques, antibiotics, surgical rubber gloves (or "boiled hands" as they were colloquially known), as well as countless procedural innovations (many of his own) that enabled surgery to become a mainstay rather than last resort option in modern medicine.
Dr. Matas earned his M.D. degree in 1880 at the Medical Department of the University of Louisiana (later Tulane University). The greater part of his career after a stint in private practice was spent as professor and chief of the department of surgery at Tulane, a post he began in 1895 and held for over 32 years, then becoming an honored emeritus.
His achievements in the surgical treatment of aneurysms began in 1888 when a manual laborer presented to him with a pulsatile swelling between his elbow and axilla two weeks after an accidental shotgun wound in the left upper arm. Unwilling to amputate or perform the standard treatment of proximal and distal ligation on a man who required both arms for his livelihood, Dr. Matas, after 3 weeks of failed attempts to thrombose the aneurysm via tourniquet or compression and then failed ligations, finally performed the endoaneurysmorrhaphy technique for which he would become famous.
According to Dr. Michael E. DeBakey, who knew Dr. Matas late in his career, the reason for his success in this and other operations was his profound curiosity. In this first aneurysm case, after he ligated both above and below the aneurysm he noticed the next day that the aneurysm was still pulsing. "He was curious as to why this happened. He couldn’t believe that his ligature had opened. So he went back in and found that his ligatures were absolutely tight. There was nothing wrong with them but the aneurysm was still pulsing. He said the only way he was going to find out was by opening it up and that is when he found the collaterals. He said it became obvious . . . to oversew the opening of these collateral vessels in the aneurysm wall, and to bring the two walls together so as to obliterate it completely. That came to him as he was operating. It was innovative in a sense, but it was his curiosity that stimulated him to do it."
The patient recovered rapidly and more than 11 years later was still gainfully employed with both arms and a palpable radial pulse.
With both tremendous modesty, and in demonstration of his profound knowledge of and interest in medical history, Dr. Matas credited Antyllus, a Greek surgeon who lived in 2nd century Rome with the original description of the surgery almost 18 centuries earlier.
Dr. Matas eventually expanded his repertoire of endoaneurysmorrhaphy to comprise obliterative, restorative, and reconstructive methodologies. The latter two techniques were modifications of the obliterative type which allowed preservation of arterial patency. He would place a catheter into the main arteries and obliterate the sac over the catheter with sutures. Versions of these techniques are still in use today.
He attempted various treatments of abdominal aortic aneurysms (AAAs), starting with use of a wire and an electric current in 1900—a failure. In 1923, he ligated the infrarenal aorta proximal to a large aneurysm—this was the first successful use of proximal ligation for an AAA.
In 1940, Dr. Matas reported on his personal experience with over 620 aneurysm operations, 101 of which were variations on the endoaneurysmorrhaphy technique. His patients had a mortality rate of less than 5%, and none of his procedures resulted in gangrene—an especially remarkable record when considering that these operations were all performed before the dawn of modern antibiotics. (Penicillin, though discovered in 1928, was not available medically until the early 1940s.) These achievements are even more remarkable when considering that Dr. Matas went permanently blind in one eye in 1908 because of to surgical splash contamination from a patient with a gonorrheal pelvic infection.
Not only were Dr. Matas’s achievements in vascular surgery profound, he was also one of the first to use spinal anesthesia; he pioneered the use of saline solutions to treat hypovolemia; he promoted operating for acute appendicitis; and he encouraged the use of nasogastric and endotracheal tubes in surgery. Dr. Matas contributed to the advancement of the surgical discipline as a whole, and in fact was one of the founders of the American College of Surgeons, which was formed, in part, to root out the practice of fee-splitting. He was one of the ACS’s first presidents.
Dr. Matas was highly revered by his colleagues and students, but he was not without his idiosyncracies. According to Dr. John Ochsner, who as a child was acquainted with the famous surgeon through his physician father, "[Dr. Matas] had one bad quality in that you could never shut him up when he started talking. . . . Dr. Matas would call and would never get off the phone, so my father would put the phone on the table and occasionally say ‘Yes, Dr. Matas,’ just to make sure he wasn’t insulting him and [he would] keep on working." And, although known as a good, if verbose lecturer (who once gave an hour and a half of remarks on a 40-minute presentation), Dr. Matas is not known for having trained large numbers of significant practitioners of academic medicine, as are many other surgical pioneers.
Dr. Matas loved to read trashy mystery novels and was a devotee of silent motion pictures from their inception. Before his death, glaucoma and cataract and a failed operation to alleviate them eliminated sight in his left eye, leaving him completely blind in 1952—the year of the first aneurysm resection of the aorta with graft replacement—the next great achievement in aneurysm surgery after his own. Dr. Matas died 5 years later, on Sept. 23, 1957, after a year of hospitalization.
"Even in the eighties (1880s), noli me tangere was written large on the head, chest and abdomen, and their contained organs were still held as in sanctuaries which no one dared to open with unhallowed hands."
—Rudolph Matas, M.D., in "Surgical Operations Fifty Years Ago" (Am. J. Surg. 1951;82:111-21)
More information about Dr. Matas can be found in the following sources:
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
Rudolph Matas, M.D, is yet another of the famous surgeons of the past 2 centuries to whom the sobriquet "father of Vascular Surgery" has been applied, primarily for his pioneering studies on and surgical treatment of aneurysms before the era of prosthetic grafts. This September is the 50th anniversary of his death.
His amazingly long career (he was born in 1860 on a Louisiana plantation and lived to age 97) spanned the period in which belief in spontaneous generation and contaminated airs and humors transformed into a new germ theory of disease. He saw the revolutions begun by Pasteur, Koch, and Lister, the development of sterilization techniques, antibiotics, surgical rubber gloves (or "boiled hands" as they were colloquially known), as well as countless procedural innovations (many of his own) that enabled surgery to become a mainstay rather than last resort option in modern medicine.
Dr. Matas earned his M.D. degree in 1880 at the Medical Department of the University of Louisiana (later Tulane University). The greater part of his career after a stint in private practice was spent as professor and chief of the department of surgery at Tulane, a post he began in 1895 and held for over 32 years, then becoming an honored emeritus.
His achievements in the surgical treatment of aneurysms began in 1888 when a manual laborer presented to him with a pulsatile swelling between his elbow and axilla two weeks after an accidental shotgun wound in the left upper arm. Unwilling to amputate or perform the standard treatment of proximal and distal ligation on a man who required both arms for his livelihood, Dr. Matas, after 3 weeks of failed attempts to thrombose the aneurysm via tourniquet or compression and then failed ligations, finally performed the endoaneurysmorrhaphy technique for which he would become famous.
According to Dr. Michael E. DeBakey, who knew Dr. Matas late in his career, the reason for his success in this and other operations was his profound curiosity. In this first aneurysm case, after he ligated both above and below the aneurysm he noticed the next day that the aneurysm was still pulsing. "He was curious as to why this happened. He couldn’t believe that his ligature had opened. So he went back in and found that his ligatures were absolutely tight. There was nothing wrong with them but the aneurysm was still pulsing. He said the only way he was going to find out was by opening it up and that is when he found the collaterals. He said it became obvious . . . to oversew the opening of these collateral vessels in the aneurysm wall, and to bring the two walls together so as to obliterate it completely. That came to him as he was operating. It was innovative in a sense, but it was his curiosity that stimulated him to do it."
The patient recovered rapidly and more than 11 years later was still gainfully employed with both arms and a palpable radial pulse.
With both tremendous modesty, and in demonstration of his profound knowledge of and interest in medical history, Dr. Matas credited Antyllus, a Greek surgeon who lived in 2nd century Rome with the original description of the surgery almost 18 centuries earlier.
Dr. Matas eventually expanded his repertoire of endoaneurysmorrhaphy to comprise obliterative, restorative, and reconstructive methodologies. The latter two techniques were modifications of the obliterative type which allowed preservation of arterial patency. He would place a catheter into the main arteries and obliterate the sac over the catheter with sutures. Versions of these techniques are still in use today.
He attempted various treatments of abdominal aortic aneurysms (AAAs), starting with use of a wire and an electric current in 1900—a failure. In 1923, he ligated the infrarenal aorta proximal to a large aneurysm—this was the first successful use of proximal ligation for an AAA.
In 1940, Dr. Matas reported on his personal experience with over 620 aneurysm operations, 101 of which were variations on the endoaneurysmorrhaphy technique. His patients had a mortality rate of less than 5%, and none of his procedures resulted in gangrene—an especially remarkable record when considering that these operations were all performed before the dawn of modern antibiotics. (Penicillin, though discovered in 1928, was not available medically until the early 1940s.) These achievements are even more remarkable when considering that Dr. Matas went permanently blind in one eye in 1908 because of to surgical splash contamination from a patient with a gonorrheal pelvic infection.
Not only were Dr. Matas’s achievements in vascular surgery profound, he was also one of the first to use spinal anesthesia; he pioneered the use of saline solutions to treat hypovolemia; he promoted operating for acute appendicitis; and he encouraged the use of nasogastric and endotracheal tubes in surgery. Dr. Matas contributed to the advancement of the surgical discipline as a whole, and in fact was one of the founders of the American College of Surgeons, which was formed, in part, to root out the practice of fee-splitting. He was one of the ACS’s first presidents.
Dr. Matas was highly revered by his colleagues and students, but he was not without his idiosyncracies. According to Dr. John Ochsner, who as a child was acquainted with the famous surgeon through his physician father, "[Dr. Matas] had one bad quality in that you could never shut him up when he started talking. . . . Dr. Matas would call and would never get off the phone, so my father would put the phone on the table and occasionally say ‘Yes, Dr. Matas,’ just to make sure he wasn’t insulting him and [he would] keep on working." And, although known as a good, if verbose lecturer (who once gave an hour and a half of remarks on a 40-minute presentation), Dr. Matas is not known for having trained large numbers of significant practitioners of academic medicine, as are many other surgical pioneers.
Dr. Matas loved to read trashy mystery novels and was a devotee of silent motion pictures from their inception. Before his death, glaucoma and cataract and a failed operation to alleviate them eliminated sight in his left eye, leaving him completely blind in 1952—the year of the first aneurysm resection of the aorta with graft replacement—the next great achievement in aneurysm surgery after his own. Dr. Matas died 5 years later, on Sept. 23, 1957, after a year of hospitalization.
"Even in the eighties (1880s), noli me tangere was written large on the head, chest and abdomen, and their contained organs were still held as in sanctuaries which no one dared to open with unhallowed hands."
—Rudolph Matas, M.D., in "Surgical Operations Fifty Years Ago" (Am. J. Surg. 1951;82:111-21)
More information about Dr. Matas can be found in the following sources:
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
Rudolph Matas, M.D, is yet another of the famous surgeons of the past 2 centuries to whom the sobriquet "father of Vascular Surgery" has been applied, primarily for his pioneering studies on and surgical treatment of aneurysms before the era of prosthetic grafts. This September is the 50th anniversary of his death.
His amazingly long career (he was born in 1860 on a Louisiana plantation and lived to age 97) spanned the period in which belief in spontaneous generation and contaminated airs and humors transformed into a new germ theory of disease. He saw the revolutions begun by Pasteur, Koch, and Lister, the development of sterilization techniques, antibiotics, surgical rubber gloves (or "boiled hands" as they were colloquially known), as well as countless procedural innovations (many of his own) that enabled surgery to become a mainstay rather than last resort option in modern medicine.
Dr. Matas earned his M.D. degree in 1880 at the Medical Department of the University of Louisiana (later Tulane University). The greater part of his career after a stint in private practice was spent as professor and chief of the department of surgery at Tulane, a post he began in 1895 and held for over 32 years, then becoming an honored emeritus.
His achievements in the surgical treatment of aneurysms began in 1888 when a manual laborer presented to him with a pulsatile swelling between his elbow and axilla two weeks after an accidental shotgun wound in the left upper arm. Unwilling to amputate or perform the standard treatment of proximal and distal ligation on a man who required both arms for his livelihood, Dr. Matas, after 3 weeks of failed attempts to thrombose the aneurysm via tourniquet or compression and then failed ligations, finally performed the endoaneurysmorrhaphy technique for which he would become famous.
According to Dr. Michael E. DeBakey, who knew Dr. Matas late in his career, the reason for his success in this and other operations was his profound curiosity. In this first aneurysm case, after he ligated both above and below the aneurysm he noticed the next day that the aneurysm was still pulsing. "He was curious as to why this happened. He couldn’t believe that his ligature had opened. So he went back in and found that his ligatures were absolutely tight. There was nothing wrong with them but the aneurysm was still pulsing. He said the only way he was going to find out was by opening it up and that is when he found the collaterals. He said it became obvious . . . to oversew the opening of these collateral vessels in the aneurysm wall, and to bring the two walls together so as to obliterate it completely. That came to him as he was operating. It was innovative in a sense, but it was his curiosity that stimulated him to do it."
The patient recovered rapidly and more than 11 years later was still gainfully employed with both arms and a palpable radial pulse.
With both tremendous modesty, and in demonstration of his profound knowledge of and interest in medical history, Dr. Matas credited Antyllus, a Greek surgeon who lived in 2nd century Rome with the original description of the surgery almost 18 centuries earlier.
Dr. Matas eventually expanded his repertoire of endoaneurysmorrhaphy to comprise obliterative, restorative, and reconstructive methodologies. The latter two techniques were modifications of the obliterative type which allowed preservation of arterial patency. He would place a catheter into the main arteries and obliterate the sac over the catheter with sutures. Versions of these techniques are still in use today.
He attempted various treatments of abdominal aortic aneurysms (AAAs), starting with use of a wire and an electric current in 1900—a failure. In 1923, he ligated the infrarenal aorta proximal to a large aneurysm—this was the first successful use of proximal ligation for an AAA.
In 1940, Dr. Matas reported on his personal experience with over 620 aneurysm operations, 101 of which were variations on the endoaneurysmorrhaphy technique. His patients had a mortality rate of less than 5%, and none of his procedures resulted in gangrene—an especially remarkable record when considering that these operations were all performed before the dawn of modern antibiotics. (Penicillin, though discovered in 1928, was not available medically until the early 1940s.) These achievements are even more remarkable when considering that Dr. Matas went permanently blind in one eye in 1908 because of to surgical splash contamination from a patient with a gonorrheal pelvic infection.
Not only were Dr. Matas’s achievements in vascular surgery profound, he was also one of the first to use spinal anesthesia; he pioneered the use of saline solutions to treat hypovolemia; he promoted operating for acute appendicitis; and he encouraged the use of nasogastric and endotracheal tubes in surgery. Dr. Matas contributed to the advancement of the surgical discipline as a whole, and in fact was one of the founders of the American College of Surgeons, which was formed, in part, to root out the practice of fee-splitting. He was one of the ACS’s first presidents.
Dr. Matas was highly revered by his colleagues and students, but he was not without his idiosyncracies. According to Dr. John Ochsner, who as a child was acquainted with the famous surgeon through his physician father, "[Dr. Matas] had one bad quality in that you could never shut him up when he started talking. . . . Dr. Matas would call and would never get off the phone, so my father would put the phone on the table and occasionally say ‘Yes, Dr. Matas,’ just to make sure he wasn’t insulting him and [he would] keep on working." And, although known as a good, if verbose lecturer (who once gave an hour and a half of remarks on a 40-minute presentation), Dr. Matas is not known for having trained large numbers of significant practitioners of academic medicine, as are many other surgical pioneers.
Dr. Matas loved to read trashy mystery novels and was a devotee of silent motion pictures from their inception. Before his death, glaucoma and cataract and a failed operation to alleviate them eliminated sight in his left eye, leaving him completely blind in 1952—the year of the first aneurysm resection of the aorta with graft replacement—the next great achievement in aneurysm surgery after his own. Dr. Matas died 5 years later, on Sept. 23, 1957, after a year of hospitalization.
"Even in the eighties (1880s), noli me tangere was written large on the head, chest and abdomen, and their contained organs were still held as in sanctuaries which no one dared to open with unhallowed hands."
—Rudolph Matas, M.D., in "Surgical Operations Fifty Years Ago" (Am. J. Surg. 1951;82:111-21)
More information about Dr. Matas can be found in the following sources:
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
E. Stanley Crawford
Ernest Stanley Crawford (1922-1992) was an internationally renowned cardiovascular surgeon whose greatest technical work involved innovative surgical techniques in the treatment of complex aortic diseases and diseases of the heart. He was a tremendous innovator who became the co-inventor of the Baylor Rapid Autologous Transfusion System, a machine that recycles a patient’s own red blood cells during surgery. The transfusion system reduced the amount of blood and blood products needed during complex aortic and other arterial surgeries, greatly increasing safety and lowering the costs of surgery. One of his specialty areas was the treatment of vascular complications of Marfan syndrome.
Dr. Crawford received his medical degree from Harvard Medical School in 1946, served in the U.S. Navy from 1947 to 1949, and completed his postgraduate surgical training at Massachusetts General Hospital. He joined the faculty at Baylor University, becoming a full professor in 1956, and remained at Baylor until the end of his career.
One of Dr. Crawford’s most important contributions to vascular surgery was the clamp-and-sew method of thoracic aortic aneurysm repair, which he developed in the 1970s. His technique involved the use of intraluminal Dacron tube grafts and the reimplantation of visceral and renal arteries. This treatment was considered to have vastly improved the efficacy of thoracic aortic aneurysm repair, but it required considerable skill on the part of the surgeon in order to avoid complications from extended cross-clamping of the aorta.
"Thousands of patients are living happy, productive lives as the result of Dr. Crawford’s contributions, both directly [he was their personal surgeon] and indirectly through his teachings to other cardiovascular surgeons," stated Dr. Cavlin B. Ernst in a memorial paper published in the Journal of Vascular Surgery in 1993, several months after Dr. Crawford’s death in October 1992.
Dr. Crawford was also intimately involved in the development of the first artificial heart assist devices, working with Dr. Michael DeBakey and Dr. Domingo Liotta at Baylor University.
"During 1961-62," Dr. Liotta reminisced, "our lab at Baylor developed a small, intrathoracic, pneumatic-driven pump that partly bypassed the left ventricle from the left atrium to the thoracic aorta....
"On 18 July 1963 one of Dr. E. Stanley Crawford’s patients underwent an aortic valve replacement. The calcified stenotic valve was replaced with a Starr-Edwards prosthetic valve. Early the next morning, the patient had a cardiac arrest and was resuscitated by means of the open-chest technique. After the chest was closed, it was evident that severe brain damage had occurred. The patient remained in a coma, with low cardiac output and anuria. Subsequently, a rather severe pulmonary edema developed and was refractory to standard treatment.
"The first clinical VAD, bypassing the left ventricle from the left atrium to the descending aorta through a left thoracotomy, was implanted in this patient on the evening of 19 July by Dr. Crawford and me. The pump was regulated to bypass with 1,800 to 2,500 mL of blood per minute. Although the anuria that had been present since cardiac arrest persisted, the pulmonary edema cleared, as indicated by plain chest x–ray and auscultation. We discontinued mechanical support after 4 days of continuous use, but the patient remained in a coma and died," said Dr. Liotta.
Among his accomplishments were 300 peer-reviewed publications and book chapters, and the much-valued textbook "Diseases of the Aorta," which he co-authored with his son, John Lloyd Crawford II, M.D.. But Dr. Crawford is perhaps most known for his contributions to professional medical associations and education.
For E. Stanley Crawford was a tireless advocate of vascular surgery as a unique and autonomous profession. As part of these efforts, Dr. Crawford made significant contributions to the evolution and development of the nuts and bolts of the Society for Vascular Surgery and the journals that serve as the bedrock of the field. He was treasurer of the Society for Vascular Surgery from 1977 to 1980 and president from 1987 to 1988, and served as a member of the editorial board of the Journal of Vascular Surgery.
Dr. Crawford was also firmly committed to vascular surgery education, and much of what the profession is today was made possible by his years of teaching and mentoring some of the finest practitioners to grace the field. He served as president of the Lifeline Foundation of the SVS, working to implement the foundation’s objective of enhancing the careers of young academic surgeons.
Among these key educational accomplishments was the development of the Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery, which today bears his name. He convened the first Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery at the SVS annual meeting in Chicago on June 11, 1988, and at the 1988 SVS Council Meeting, under his aegis, it was decided that one of the responsibilities of the president-elect would be to organize and orchestrate future Critical Issues meetings.
Sources and Suggested Readings
1) J. Vasc. Surg. 1996;23:1081-7.
2) J. Vasc. Surg. 1993;17:618-19.
3) Tex. Heart Inst. J. 2002;29:229-30.
4) Vascular Surgery Principles and Practice, 3rd ed.; Robert W. Hobson II, Samuel E. Wilson, Frank J. Veith, Eds.; Marcel Dekker: New York, 2003.
Ernest Stanley Crawford (1922-1992) was an internationally renowned cardiovascular surgeon whose greatest technical work involved innovative surgical techniques in the treatment of complex aortic diseases and diseases of the heart. He was a tremendous innovator who became the co-inventor of the Baylor Rapid Autologous Transfusion System, a machine that recycles a patient’s own red blood cells during surgery. The transfusion system reduced the amount of blood and blood products needed during complex aortic and other arterial surgeries, greatly increasing safety and lowering the costs of surgery. One of his specialty areas was the treatment of vascular complications of Marfan syndrome.
Dr. Crawford received his medical degree from Harvard Medical School in 1946, served in the U.S. Navy from 1947 to 1949, and completed his postgraduate surgical training at Massachusetts General Hospital. He joined the faculty at Baylor University, becoming a full professor in 1956, and remained at Baylor until the end of his career.
One of Dr. Crawford’s most important contributions to vascular surgery was the clamp-and-sew method of thoracic aortic aneurysm repair, which he developed in the 1970s. His technique involved the use of intraluminal Dacron tube grafts and the reimplantation of visceral and renal arteries. This treatment was considered to have vastly improved the efficacy of thoracic aortic aneurysm repair, but it required considerable skill on the part of the surgeon in order to avoid complications from extended cross-clamping of the aorta.
"Thousands of patients are living happy, productive lives as the result of Dr. Crawford’s contributions, both directly [he was their personal surgeon] and indirectly through his teachings to other cardiovascular surgeons," stated Dr. Cavlin B. Ernst in a memorial paper published in the Journal of Vascular Surgery in 1993, several months after Dr. Crawford’s death in October 1992.
Dr. Crawford was also intimately involved in the development of the first artificial heart assist devices, working with Dr. Michael DeBakey and Dr. Domingo Liotta at Baylor University.
"During 1961-62," Dr. Liotta reminisced, "our lab at Baylor developed a small, intrathoracic, pneumatic-driven pump that partly bypassed the left ventricle from the left atrium to the thoracic aorta....
"On 18 July 1963 one of Dr. E. Stanley Crawford’s patients underwent an aortic valve replacement. The calcified stenotic valve was replaced with a Starr-Edwards prosthetic valve. Early the next morning, the patient had a cardiac arrest and was resuscitated by means of the open-chest technique. After the chest was closed, it was evident that severe brain damage had occurred. The patient remained in a coma, with low cardiac output and anuria. Subsequently, a rather severe pulmonary edema developed and was refractory to standard treatment.
"The first clinical VAD, bypassing the left ventricle from the left atrium to the descending aorta through a left thoracotomy, was implanted in this patient on the evening of 19 July by Dr. Crawford and me. The pump was regulated to bypass with 1,800 to 2,500 mL of blood per minute. Although the anuria that had been present since cardiac arrest persisted, the pulmonary edema cleared, as indicated by plain chest x–ray and auscultation. We discontinued mechanical support after 4 days of continuous use, but the patient remained in a coma and died," said Dr. Liotta.
Among his accomplishments were 300 peer-reviewed publications and book chapters, and the much-valued textbook "Diseases of the Aorta," which he co-authored with his son, John Lloyd Crawford II, M.D.. But Dr. Crawford is perhaps most known for his contributions to professional medical associations and education.
For E. Stanley Crawford was a tireless advocate of vascular surgery as a unique and autonomous profession. As part of these efforts, Dr. Crawford made significant contributions to the evolution and development of the nuts and bolts of the Society for Vascular Surgery and the journals that serve as the bedrock of the field. He was treasurer of the Society for Vascular Surgery from 1977 to 1980 and president from 1987 to 1988, and served as a member of the editorial board of the Journal of Vascular Surgery.
Dr. Crawford was also firmly committed to vascular surgery education, and much of what the profession is today was made possible by his years of teaching and mentoring some of the finest practitioners to grace the field. He served as president of the Lifeline Foundation of the SVS, working to implement the foundation’s objective of enhancing the careers of young academic surgeons.
Among these key educational accomplishments was the development of the Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery, which today bears his name. He convened the first Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery at the SVS annual meeting in Chicago on June 11, 1988, and at the 1988 SVS Council Meeting, under his aegis, it was decided that one of the responsibilities of the president-elect would be to organize and orchestrate future Critical Issues meetings.
Sources and Suggested Readings
1) J. Vasc. Surg. 1996;23:1081-7.
2) J. Vasc. Surg. 1993;17:618-19.
3) Tex. Heart Inst. J. 2002;29:229-30.
4) Vascular Surgery Principles and Practice, 3rd ed.; Robert W. Hobson II, Samuel E. Wilson, Frank J. Veith, Eds.; Marcel Dekker: New York, 2003.
Ernest Stanley Crawford (1922-1992) was an internationally renowned cardiovascular surgeon whose greatest technical work involved innovative surgical techniques in the treatment of complex aortic diseases and diseases of the heart. He was a tremendous innovator who became the co-inventor of the Baylor Rapid Autologous Transfusion System, a machine that recycles a patient’s own red blood cells during surgery. The transfusion system reduced the amount of blood and blood products needed during complex aortic and other arterial surgeries, greatly increasing safety and lowering the costs of surgery. One of his specialty areas was the treatment of vascular complications of Marfan syndrome.
Dr. Crawford received his medical degree from Harvard Medical School in 1946, served in the U.S. Navy from 1947 to 1949, and completed his postgraduate surgical training at Massachusetts General Hospital. He joined the faculty at Baylor University, becoming a full professor in 1956, and remained at Baylor until the end of his career.
One of Dr. Crawford’s most important contributions to vascular surgery was the clamp-and-sew method of thoracic aortic aneurysm repair, which he developed in the 1970s. His technique involved the use of intraluminal Dacron tube grafts and the reimplantation of visceral and renal arteries. This treatment was considered to have vastly improved the efficacy of thoracic aortic aneurysm repair, but it required considerable skill on the part of the surgeon in order to avoid complications from extended cross-clamping of the aorta.
"Thousands of patients are living happy, productive lives as the result of Dr. Crawford’s contributions, both directly [he was their personal surgeon] and indirectly through his teachings to other cardiovascular surgeons," stated Dr. Cavlin B. Ernst in a memorial paper published in the Journal of Vascular Surgery in 1993, several months after Dr. Crawford’s death in October 1992.
Dr. Crawford was also intimately involved in the development of the first artificial heart assist devices, working with Dr. Michael DeBakey and Dr. Domingo Liotta at Baylor University.
"During 1961-62," Dr. Liotta reminisced, "our lab at Baylor developed a small, intrathoracic, pneumatic-driven pump that partly bypassed the left ventricle from the left atrium to the thoracic aorta....
"On 18 July 1963 one of Dr. E. Stanley Crawford’s patients underwent an aortic valve replacement. The calcified stenotic valve was replaced with a Starr-Edwards prosthetic valve. Early the next morning, the patient had a cardiac arrest and was resuscitated by means of the open-chest technique. After the chest was closed, it was evident that severe brain damage had occurred. The patient remained in a coma, with low cardiac output and anuria. Subsequently, a rather severe pulmonary edema developed and was refractory to standard treatment.
"The first clinical VAD, bypassing the left ventricle from the left atrium to the descending aorta through a left thoracotomy, was implanted in this patient on the evening of 19 July by Dr. Crawford and me. The pump was regulated to bypass with 1,800 to 2,500 mL of blood per minute. Although the anuria that had been present since cardiac arrest persisted, the pulmonary edema cleared, as indicated by plain chest x–ray and auscultation. We discontinued mechanical support after 4 days of continuous use, but the patient remained in a coma and died," said Dr. Liotta.
Among his accomplishments were 300 peer-reviewed publications and book chapters, and the much-valued textbook "Diseases of the Aorta," which he co-authored with his son, John Lloyd Crawford II, M.D.. But Dr. Crawford is perhaps most known for his contributions to professional medical associations and education.
For E. Stanley Crawford was a tireless advocate of vascular surgery as a unique and autonomous profession. As part of these efforts, Dr. Crawford made significant contributions to the evolution and development of the nuts and bolts of the Society for Vascular Surgery and the journals that serve as the bedrock of the field. He was treasurer of the Society for Vascular Surgery from 1977 to 1980 and president from 1987 to 1988, and served as a member of the editorial board of the Journal of Vascular Surgery.
Dr. Crawford was also firmly committed to vascular surgery education, and much of what the profession is today was made possible by his years of teaching and mentoring some of the finest practitioners to grace the field. He served as president of the Lifeline Foundation of the SVS, working to implement the foundation’s objective of enhancing the careers of young academic surgeons.
Among these key educational accomplishments was the development of the Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery, which today bears his name. He convened the first Society for Vascular Surgery Forum on Critical Issues in Vascular Surgery at the SVS annual meeting in Chicago on June 11, 1988, and at the 1988 SVS Council Meeting, under his aegis, it was decided that one of the responsibilities of the president-elect would be to organize and orchestrate future Critical Issues meetings.
Sources and Suggested Readings
1) J. Vasc. Surg. 1996;23:1081-7.
2) J. Vasc. Surg. 1993;17:618-19.
3) Tex. Heart Inst. J. 2002;29:229-30.
4) Vascular Surgery Principles and Practice, 3rd ed.; Robert W. Hobson II, Samuel E. Wilson, Frank J. Veith, Eds.; Marcel Dekker: New York, 2003.
John Hunter and the Vasculature
"If Hunter were to return to life, nothing, I believe, would grieve him so much as the fact that tying the femoral artery where its coats are sound for the treatment of popliteal aneurysm is counted one of ‘his major contributions to surgery.’ That operation was merely a side issue in a great chain of discoveries that revealed the nature of the vascular system. Hunter was the first to discover the mechanism of arteries. He demonstrated that they had an inherent and independent life and action." — Arthur Keith, M.D., in What Did John Hunter Do for Medicine?
John Hunter, born in 1728, was the brother of the great anatomist William Hunter (1718-1783), whose interest in vascular surgery was easily transmitted to his younger sibling. He achieved surgical mastery in an era when the application of science to medicine was in its thriving infancy.
William Hunter was best known in vascular surgery for his identification of arterial-venous communication as an atypical aneurysm, most often caused by the then common practice of venesection. In 1757, William Hunter published "The History of an Aneurysm of the Aorta with Some Remarks on Aneurysms in General," in which he added the category of "mixed" aneurysm to those of true (spontaneous) and false (traumatic). Mixed aneurysms, he stated, were caused by a wound or rupture of the coats of the artery and partly by a dilatation of the rest. William was also responsible for reporting the Lambert and Hallowell repair of a lacerated brachial artery, which "would stimulate the first concerted effort to repair arteries more than a century later," according to Dr. Steven G. Friedman in "A History of Vascular Surgery."
At the age of 20, John Hunter joined his brother in London at William’s Covent Garden Anatomy School, where he became proficient in anatomy and dissection. He then went on to study with Dr. Percival Pott of St. Bartholomew’s Hospital and became the master of anatomy at Surgeon’s Hall. As house surgeon at St. George’s Hospital from 1754 to 1756, he made some of his most important discoveries, including descriptions of the lymphatic vessels in birds and the circulation of the uterus and the placenta.
In 1760, he joined the British army fighting in Portugal during the Seven Years’ War, becoming one of the early examples of vascular surgeons who developed their skills and observations during military service. Many ascribe this experience as the groundwork for his famous work published posthumously in 1794, "A Treatise on Blood, Inflammation, and Gun-Shot Wounds." In this work Hunter provided not only the first comprehensive mechanistic framework for the inflammatory response, but also appears to be the first person to discover the connection between the erythrocyte sedimentation rate (ESR) and inflammation (blood cells sedimented faster during inflammation). He also noted that the change in ESR was not restricted to the blood in the local area of the inflammation, but was systemic. With such observations, Hunter was one of the strongest supporters of blood as a living, changeable entity.
In 1767, John Hunter was elected a Fellow of the Royal Society and a member of the Corporation of Surgeons. Hunter was responsible for teaching some of the most important physicians and surgeons of the next generation, including Edward Jenner, the father of vaccination.
One of the things that Hunter is credited for—the discovery of collateral circulation via an experiment on stag horns—is not tenable, given the fact that such collateral circulation was already known by his contemporaries. According to some historians, however, it is probable that his recognition of the significance of collateral circulation in preserving the vitality of a deer’s antlers after ligation of the external carotid artery may have proved inspirational in his treatment of aneurysms.
John Hunter’s most famous operation occurred on December 12, 1785, when he successfully treated a 45-year-old coachman suffering from a large spontaneous popliteal aneurysm by ligating the superficial femoral artery at the distal end of the subsartorial canal, leaving the aneurysm intact. This "departed radically from the teaching of two thousand years," according to an article published in honor of the bicentennial of his operation. Traditional treatment promulgated by the Greek, Antyllus, in the 3rd century B.C. consisted of ligation of the artery above and below the lesion and then evacuation of its contents. Collateral circulation maintained the viability of the limb.
In the era in which Hunter operated, techniques for this treatment were often crude; ligatures often included surrounding structures, and were coarse, wide, and had ends left hanging from the wounds. It was expected that purulent ligatures would ultimately be expelled. In addition, the tremendous lesion produced by removing the often enormous aneurysm was another source of infection. The success rate, or rather lack thereof, can be imagined.
In fact, Dr. Percival Potts, Hunter’s early mentor, believed that treating popliteal aneurysms was futile, and Dr. Bradford Wilmer stated in 1779 that "with regard to aneurysm of the popliteal artery, there is not, that I know, a single case upon record where that operation has succeeded." Amputation, where possible, was considered by many to be the only real solution.
However, Hunter’s patient not only survived but thrived for 15 more months, at which point he died of a "remittent fever." Hunter performed a necropsy on the treated limb and found it was entirely free from putrefaction.
Hunter performed his operation on four more patients between 1787 and 1793, with 3 successes and 1 failure. Surprisingly, Hunter thought so little of the operation compared with his other accomplishments that he never published on it himself. Instead, the technique was promulgated by his brother-in-law and assistant, Dr. Everard Home, in several publications.
Hunter’s operation was supplanted by the advent of Dr. Rudolph Matas’ endoaneurysmorrhaphy treatment for aneurysms a century later. In that era, antiseptic methods became more readily available and the feasibility of emptying or ablating the aneurysm sac once more became viable.
John Hunter died in 1793. He was the victim of a syphilitic ascending aortic aneurysm, a condition resulting from his earlier investigations into venereal disease. In 1767 he self-inoculated his penis with a specimen taken from a patient with gonorrheal urethritis who also, unbeknownst to him, carried syphilis, for which there was then no cure.
More information about John Hunter can be found in the following sources used for this article:
• "John Hunter, Velvet and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1984;66:214-18).
• "John Hunter and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1995;Spec. No. 26-31).
• "Popliteal Aneurysm: A Celebration of the Bicentennial of John Hunter’s Operation" (Ann. Vasc. Surg. 1986;1:118-26).
• "Giving Credit Where Credit is Due: John Hunter and the Discovery of Erythrocyte Sedimentation Rate" (Lancet 2005;366:2140-41).
• "What did John Hunter do For Medicine?" (Brit. Med. J. 1919;2:485-87).
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
"If Hunter were to return to life, nothing, I believe, would grieve him so much as the fact that tying the femoral artery where its coats are sound for the treatment of popliteal aneurysm is counted one of ‘his major contributions to surgery.’ That operation was merely a side issue in a great chain of discoveries that revealed the nature of the vascular system. Hunter was the first to discover the mechanism of arteries. He demonstrated that they had an inherent and independent life and action." — Arthur Keith, M.D., in What Did John Hunter Do for Medicine?
John Hunter, born in 1728, was the brother of the great anatomist William Hunter (1718-1783), whose interest in vascular surgery was easily transmitted to his younger sibling. He achieved surgical mastery in an era when the application of science to medicine was in its thriving infancy.
William Hunter was best known in vascular surgery for his identification of arterial-venous communication as an atypical aneurysm, most often caused by the then common practice of venesection. In 1757, William Hunter published "The History of an Aneurysm of the Aorta with Some Remarks on Aneurysms in General," in which he added the category of "mixed" aneurysm to those of true (spontaneous) and false (traumatic). Mixed aneurysms, he stated, were caused by a wound or rupture of the coats of the artery and partly by a dilatation of the rest. William was also responsible for reporting the Lambert and Hallowell repair of a lacerated brachial artery, which "would stimulate the first concerted effort to repair arteries more than a century later," according to Dr. Steven G. Friedman in "A History of Vascular Surgery."
At the age of 20, John Hunter joined his brother in London at William’s Covent Garden Anatomy School, where he became proficient in anatomy and dissection. He then went on to study with Dr. Percival Pott of St. Bartholomew’s Hospital and became the master of anatomy at Surgeon’s Hall. As house surgeon at St. George’s Hospital from 1754 to 1756, he made some of his most important discoveries, including descriptions of the lymphatic vessels in birds and the circulation of the uterus and the placenta.
In 1760, he joined the British army fighting in Portugal during the Seven Years’ War, becoming one of the early examples of vascular surgeons who developed their skills and observations during military service. Many ascribe this experience as the groundwork for his famous work published posthumously in 1794, "A Treatise on Blood, Inflammation, and Gun-Shot Wounds." In this work Hunter provided not only the first comprehensive mechanistic framework for the inflammatory response, but also appears to be the first person to discover the connection between the erythrocyte sedimentation rate (ESR) and inflammation (blood cells sedimented faster during inflammation). He also noted that the change in ESR was not restricted to the blood in the local area of the inflammation, but was systemic. With such observations, Hunter was one of the strongest supporters of blood as a living, changeable entity.
In 1767, John Hunter was elected a Fellow of the Royal Society and a member of the Corporation of Surgeons. Hunter was responsible for teaching some of the most important physicians and surgeons of the next generation, including Edward Jenner, the father of vaccination.
One of the things that Hunter is credited for—the discovery of collateral circulation via an experiment on stag horns—is not tenable, given the fact that such collateral circulation was already known by his contemporaries. According to some historians, however, it is probable that his recognition of the significance of collateral circulation in preserving the vitality of a deer’s antlers after ligation of the external carotid artery may have proved inspirational in his treatment of aneurysms.
John Hunter’s most famous operation occurred on December 12, 1785, when he successfully treated a 45-year-old coachman suffering from a large spontaneous popliteal aneurysm by ligating the superficial femoral artery at the distal end of the subsartorial canal, leaving the aneurysm intact. This "departed radically from the teaching of two thousand years," according to an article published in honor of the bicentennial of his operation. Traditional treatment promulgated by the Greek, Antyllus, in the 3rd century B.C. consisted of ligation of the artery above and below the lesion and then evacuation of its contents. Collateral circulation maintained the viability of the limb.
In the era in which Hunter operated, techniques for this treatment were often crude; ligatures often included surrounding structures, and were coarse, wide, and had ends left hanging from the wounds. It was expected that purulent ligatures would ultimately be expelled. In addition, the tremendous lesion produced by removing the often enormous aneurysm was another source of infection. The success rate, or rather lack thereof, can be imagined.
In fact, Dr. Percival Potts, Hunter’s early mentor, believed that treating popliteal aneurysms was futile, and Dr. Bradford Wilmer stated in 1779 that "with regard to aneurysm of the popliteal artery, there is not, that I know, a single case upon record where that operation has succeeded." Amputation, where possible, was considered by many to be the only real solution.
However, Hunter’s patient not only survived but thrived for 15 more months, at which point he died of a "remittent fever." Hunter performed a necropsy on the treated limb and found it was entirely free from putrefaction.
Hunter performed his operation on four more patients between 1787 and 1793, with 3 successes and 1 failure. Surprisingly, Hunter thought so little of the operation compared with his other accomplishments that he never published on it himself. Instead, the technique was promulgated by his brother-in-law and assistant, Dr. Everard Home, in several publications.
Hunter’s operation was supplanted by the advent of Dr. Rudolph Matas’ endoaneurysmorrhaphy treatment for aneurysms a century later. In that era, antiseptic methods became more readily available and the feasibility of emptying or ablating the aneurysm sac once more became viable.
John Hunter died in 1793. He was the victim of a syphilitic ascending aortic aneurysm, a condition resulting from his earlier investigations into venereal disease. In 1767 he self-inoculated his penis with a specimen taken from a patient with gonorrheal urethritis who also, unbeknownst to him, carried syphilis, for which there was then no cure.
More information about John Hunter can be found in the following sources used for this article:
• "John Hunter, Velvet and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1984;66:214-18).
• "John Hunter and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1995;Spec. No. 26-31).
• "Popliteal Aneurysm: A Celebration of the Bicentennial of John Hunter’s Operation" (Ann. Vasc. Surg. 1986;1:118-26).
• "Giving Credit Where Credit is Due: John Hunter and the Discovery of Erythrocyte Sedimentation Rate" (Lancet 2005;366:2140-41).
• "What did John Hunter do For Medicine?" (Brit. Med. J. 1919;2:485-87).
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
"If Hunter were to return to life, nothing, I believe, would grieve him so much as the fact that tying the femoral artery where its coats are sound for the treatment of popliteal aneurysm is counted one of ‘his major contributions to surgery.’ That operation was merely a side issue in a great chain of discoveries that revealed the nature of the vascular system. Hunter was the first to discover the mechanism of arteries. He demonstrated that they had an inherent and independent life and action." — Arthur Keith, M.D., in What Did John Hunter Do for Medicine?
John Hunter, born in 1728, was the brother of the great anatomist William Hunter (1718-1783), whose interest in vascular surgery was easily transmitted to his younger sibling. He achieved surgical mastery in an era when the application of science to medicine was in its thriving infancy.
William Hunter was best known in vascular surgery for his identification of arterial-venous communication as an atypical aneurysm, most often caused by the then common practice of venesection. In 1757, William Hunter published "The History of an Aneurysm of the Aorta with Some Remarks on Aneurysms in General," in which he added the category of "mixed" aneurysm to those of true (spontaneous) and false (traumatic). Mixed aneurysms, he stated, were caused by a wound or rupture of the coats of the artery and partly by a dilatation of the rest. William was also responsible for reporting the Lambert and Hallowell repair of a lacerated brachial artery, which "would stimulate the first concerted effort to repair arteries more than a century later," according to Dr. Steven G. Friedman in "A History of Vascular Surgery."
At the age of 20, John Hunter joined his brother in London at William’s Covent Garden Anatomy School, where he became proficient in anatomy and dissection. He then went on to study with Dr. Percival Pott of St. Bartholomew’s Hospital and became the master of anatomy at Surgeon’s Hall. As house surgeon at St. George’s Hospital from 1754 to 1756, he made some of his most important discoveries, including descriptions of the lymphatic vessels in birds and the circulation of the uterus and the placenta.
In 1760, he joined the British army fighting in Portugal during the Seven Years’ War, becoming one of the early examples of vascular surgeons who developed their skills and observations during military service. Many ascribe this experience as the groundwork for his famous work published posthumously in 1794, "A Treatise on Blood, Inflammation, and Gun-Shot Wounds." In this work Hunter provided not only the first comprehensive mechanistic framework for the inflammatory response, but also appears to be the first person to discover the connection between the erythrocyte sedimentation rate (ESR) and inflammation (blood cells sedimented faster during inflammation). He also noted that the change in ESR was not restricted to the blood in the local area of the inflammation, but was systemic. With such observations, Hunter was one of the strongest supporters of blood as a living, changeable entity.
In 1767, John Hunter was elected a Fellow of the Royal Society and a member of the Corporation of Surgeons. Hunter was responsible for teaching some of the most important physicians and surgeons of the next generation, including Edward Jenner, the father of vaccination.
One of the things that Hunter is credited for—the discovery of collateral circulation via an experiment on stag horns—is not tenable, given the fact that such collateral circulation was already known by his contemporaries. According to some historians, however, it is probable that his recognition of the significance of collateral circulation in preserving the vitality of a deer’s antlers after ligation of the external carotid artery may have proved inspirational in his treatment of aneurysms.
John Hunter’s most famous operation occurred on December 12, 1785, when he successfully treated a 45-year-old coachman suffering from a large spontaneous popliteal aneurysm by ligating the superficial femoral artery at the distal end of the subsartorial canal, leaving the aneurysm intact. This "departed radically from the teaching of two thousand years," according to an article published in honor of the bicentennial of his operation. Traditional treatment promulgated by the Greek, Antyllus, in the 3rd century B.C. consisted of ligation of the artery above and below the lesion and then evacuation of its contents. Collateral circulation maintained the viability of the limb.
In the era in which Hunter operated, techniques for this treatment were often crude; ligatures often included surrounding structures, and were coarse, wide, and had ends left hanging from the wounds. It was expected that purulent ligatures would ultimately be expelled. In addition, the tremendous lesion produced by removing the often enormous aneurysm was another source of infection. The success rate, or rather lack thereof, can be imagined.
In fact, Dr. Percival Potts, Hunter’s early mentor, believed that treating popliteal aneurysms was futile, and Dr. Bradford Wilmer stated in 1779 that "with regard to aneurysm of the popliteal artery, there is not, that I know, a single case upon record where that operation has succeeded." Amputation, where possible, was considered by many to be the only real solution.
However, Hunter’s patient not only survived but thrived for 15 more months, at which point he died of a "remittent fever." Hunter performed a necropsy on the treated limb and found it was entirely free from putrefaction.
Hunter performed his operation on four more patients between 1787 and 1793, with 3 successes and 1 failure. Surprisingly, Hunter thought so little of the operation compared with his other accomplishments that he never published on it himself. Instead, the technique was promulgated by his brother-in-law and assistant, Dr. Everard Home, in several publications.
Hunter’s operation was supplanted by the advent of Dr. Rudolph Matas’ endoaneurysmorrhaphy treatment for aneurysms a century later. In that era, antiseptic methods became more readily available and the feasibility of emptying or ablating the aneurysm sac once more became viable.
John Hunter died in 1793. He was the victim of a syphilitic ascending aortic aneurysm, a condition resulting from his earlier investigations into venereal disease. In 1767 he self-inoculated his penis with a specimen taken from a patient with gonorrheal urethritis who also, unbeknownst to him, carried syphilis, for which there was then no cure.
More information about John Hunter can be found in the following sources used for this article:
• "John Hunter, Velvet and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1984;66:214-18).
• "John Hunter and Vascular Surgery" (Ann. R. Coll. Surg. Engl. 1995;Spec. No. 26-31).
• "Popliteal Aneurysm: A Celebration of the Bicentennial of John Hunter’s Operation" (Ann. Vasc. Surg. 1986;1:118-26).
• "Giving Credit Where Credit is Due: John Hunter and the Discovery of Erythrocyte Sedimentation Rate" (Lancet 2005;366:2140-41).
• "What did John Hunter do For Medicine?" (Brit. Med. J. 1919;2:485-87).
• "A History of Vascular Surgery" by Steven G. Friedman, M.D. (Mount Kisco, N.Y.: Futura Publishing Co. 1989).
Michael E. DeBakey
Dr. Michael Ellis DeBakey, pioneer heart surgeon and medical device innovator, died July 11, 2008, in Houston, about 2 months shy of his 100th birthday on Sept. 7.
In his lifetime, Dr. DeBakey was renowned for his immense contributions to the progress of medical science, such that he was declared a "living legend" by the Library of Congress and was this year awarded a Congressional Gold Medal for his lifetime achievements, in particular his pioneering work as a heart surgeon.
And although it was as a heart surgeon that Dr. DeBakey gained greatest fame, his accomplisments in vascular surgery were unique and profound, from his development of the Dacron graft to his active membership in the SVS, and his role as founder and first editor of the Journal of Vascular Surgery.
Even before Dr. DeBakey received his medical degree from Tulane University in 1932, he began his contributions to modern medicine by developing a small continuous flow–roller pump designed to improve blood transfusion—a device that would later be used by Dr. John Gibbon as a crucial component of his heart-lung machine. And in 1939, with his mentor, Dr. Alton Ochsner, Dr. DeBakey suggested a strong link between smoking and lung cancer.
After internships in New Orleans and surgical training in Europe, Dr. DeBakey volunteered for service during World War II and was assigned to the U.S. Army Surgeon General’s office. From his observations in the field, he became convinced of the need for a mobile surgical unit that would give soldiers access to high-level medical treatment on the combat field and convinced the surgeon general to form what would become the mobile army surgical hospitals (MASH units)—an innovation that gained him the U.S. Army Legion of Merit in 1945.
His government service continued throughout his civilian career, as he helped to establish the Veterans Administration medical center research system. He also initiated the movement that in 1956 took the Army’s poorly housed medical library and used it to create the National Library of Medicine, of which he was first board member and then chairman. He served three terms on the National Heart, Lung, and Blood Advisory Council as well. He was responsible for helping establish health care systems in a host of countries, including Belgium, China, Egypt, England, Germany, Saudi Arabia, Australia, and numerous other Middle Eastern and Central and South American nations.
According to the Web site of Baylor College of Medicine’s department of surgery, where he spent almost his entire postwar career, Dr. DeBakey operated on more than 60,000 patients in the Houston area alone. But these were not all just standard operations. In 1953, he performed the first successful carotid endarterectomy, as well as the first successful removal and graft replacement of a fusiform thoracic aortic aneurysm, and in 1954, the first successful resection and graft replacement of an aneurysm of the distal aortic arch and upper descending thoracic aorta.
In 1955 he performed the first successful resection of a thoracoabdominal aortic aneurysm using the DeBakey Dacron graft—the first artificial arterial graft of its kind.
"If we now tried to develop the Dacron graft the way we developed it, I am not sure we would have it today with the way they regulate things. ...When I went down to the department store . . . they said, ‘We are fresh out of nylon, but we do have a new material called Dacron.’ I felt it, and it looked good to me. So I bought a yard of it. . . . I took this yard of Dacron cloth, I cut two sheets the width I wanted, sewed the edges on each side, and made a tube out of it. . . . We put the graft on a stent, wrapped nylon thread around it, pushed it together, and baked it. . . . After about two or three years of laboratory work on my own [including experiments in dogs], I decided that it was time to put the graft in a human being. I did not have a committee to approve it. . . . In 1954, I put the first one in during an abdominal aortic aneurysm. That first patient lived, I think, for 13 years and never had any trouble," Dr. DeBakey related in an interview published in 1996 in the Journal of Vascular Surgery.
And among his other pioneering surgical developments, in 1964, Dr. DeBakey was the first to perform a successful coronary artery bypass, using a portion of leg vein as the graft, in what is now one of the most commonly performed heart operations—coronary artery bypass grafting.
As if surgically repairing failing hearts was not enough, Dr. DeBakey became a pioneer of artificial heart research and of cardiac assist devices. On July 18, 1963, after years of animal research, he performed the first successful human implantation of a left ventricular assist device (LVAD), one which he devised; the patient died after 4 days from causes unrelated to the technology. In 1966, Dr. DeBakey’s redesigned, extracorporeal pneumatic pump was used in a 37-year-old woman who could not be weaned from the heart-lung machine after dual valve replacement. After 10 days of LVAD support, she recovered sufficiently for the pump to be removed and she survived. This pump served as the basis of Dr. DeBakey’s first total artificial heart model, created in 1968.
Dr. DeBakey was honored profusely throughout his lifetime by the medical community and the general public. Numerous medical facilities are named after him in this country and around the world. He received countless awards for his technical and social achievements in medicine. Among these honors were the American Medical Association’s Distinguished Service Award (1959), the Albert Lasker Award for Clinical Medical Research (1963), the Presidential Medal of Freedom (1969), and the National Medal of Science (1987). More recently, he was the first foreign member elected to the Russian Academy of Sciences (1999), was given the Library of Congress Bicentennial Living Legend Award (2000), and was awarded the Congressional Gold Medal in April 2008.
In his death, Dr. DeBakey was the first Houston resident given the honor of lying in state at City Hall and, at the request of his family, he lay dressed in his characteristic glasses, scrubs, and white coat for viewing by long lines of the general public.n
Sources and suggested readingsHeart Fail. Clin. 2007;3:117-20.J. Vasc. Surg. 1996;23:1031-4.
Dr. Michael Ellis DeBakey, pioneer heart surgeon and medical device innovator, died July 11, 2008, in Houston, about 2 months shy of his 100th birthday on Sept. 7.
In his lifetime, Dr. DeBakey was renowned for his immense contributions to the progress of medical science, such that he was declared a "living legend" by the Library of Congress and was this year awarded a Congressional Gold Medal for his lifetime achievements, in particular his pioneering work as a heart surgeon.
And although it was as a heart surgeon that Dr. DeBakey gained greatest fame, his accomplisments in vascular surgery were unique and profound, from his development of the Dacron graft to his active membership in the SVS, and his role as founder and first editor of the Journal of Vascular Surgery.
Even before Dr. DeBakey received his medical degree from Tulane University in 1932, he began his contributions to modern medicine by developing a small continuous flow–roller pump designed to improve blood transfusion—a device that would later be used by Dr. John Gibbon as a crucial component of his heart-lung machine. And in 1939, with his mentor, Dr. Alton Ochsner, Dr. DeBakey suggested a strong link between smoking and lung cancer.
After internships in New Orleans and surgical training in Europe, Dr. DeBakey volunteered for service during World War II and was assigned to the U.S. Army Surgeon General’s office. From his observations in the field, he became convinced of the need for a mobile surgical unit that would give soldiers access to high-level medical treatment on the combat field and convinced the surgeon general to form what would become the mobile army surgical hospitals (MASH units)—an innovation that gained him the U.S. Army Legion of Merit in 1945.
His government service continued throughout his civilian career, as he helped to establish the Veterans Administration medical center research system. He also initiated the movement that in 1956 took the Army’s poorly housed medical library and used it to create the National Library of Medicine, of which he was first board member and then chairman. He served three terms on the National Heart, Lung, and Blood Advisory Council as well. He was responsible for helping establish health care systems in a host of countries, including Belgium, China, Egypt, England, Germany, Saudi Arabia, Australia, and numerous other Middle Eastern and Central and South American nations.
According to the Web site of Baylor College of Medicine’s department of surgery, where he spent almost his entire postwar career, Dr. DeBakey operated on more than 60,000 patients in the Houston area alone. But these were not all just standard operations. In 1953, he performed the first successful carotid endarterectomy, as well as the first successful removal and graft replacement of a fusiform thoracic aortic aneurysm, and in 1954, the first successful resection and graft replacement of an aneurysm of the distal aortic arch and upper descending thoracic aorta.
In 1955 he performed the first successful resection of a thoracoabdominal aortic aneurysm using the DeBakey Dacron graft—the first artificial arterial graft of its kind.
"If we now tried to develop the Dacron graft the way we developed it, I am not sure we would have it today with the way they regulate things. ...When I went down to the department store . . . they said, ‘We are fresh out of nylon, but we do have a new material called Dacron.’ I felt it, and it looked good to me. So I bought a yard of it. . . . I took this yard of Dacron cloth, I cut two sheets the width I wanted, sewed the edges on each side, and made a tube out of it. . . . We put the graft on a stent, wrapped nylon thread around it, pushed it together, and baked it. . . . After about two or three years of laboratory work on my own [including experiments in dogs], I decided that it was time to put the graft in a human being. I did not have a committee to approve it. . . . In 1954, I put the first one in during an abdominal aortic aneurysm. That first patient lived, I think, for 13 years and never had any trouble," Dr. DeBakey related in an interview published in 1996 in the Journal of Vascular Surgery.
And among his other pioneering surgical developments, in 1964, Dr. DeBakey was the first to perform a successful coronary artery bypass, using a portion of leg vein as the graft, in what is now one of the most commonly performed heart operations—coronary artery bypass grafting.
As if surgically repairing failing hearts was not enough, Dr. DeBakey became a pioneer of artificial heart research and of cardiac assist devices. On July 18, 1963, after years of animal research, he performed the first successful human implantation of a left ventricular assist device (LVAD), one which he devised; the patient died after 4 days from causes unrelated to the technology. In 1966, Dr. DeBakey’s redesigned, extracorporeal pneumatic pump was used in a 37-year-old woman who could not be weaned from the heart-lung machine after dual valve replacement. After 10 days of LVAD support, she recovered sufficiently for the pump to be removed and she survived. This pump served as the basis of Dr. DeBakey’s first total artificial heart model, created in 1968.
Dr. DeBakey was honored profusely throughout his lifetime by the medical community and the general public. Numerous medical facilities are named after him in this country and around the world. He received countless awards for his technical and social achievements in medicine. Among these honors were the American Medical Association’s Distinguished Service Award (1959), the Albert Lasker Award for Clinical Medical Research (1963), the Presidential Medal of Freedom (1969), and the National Medal of Science (1987). More recently, he was the first foreign member elected to the Russian Academy of Sciences (1999), was given the Library of Congress Bicentennial Living Legend Award (2000), and was awarded the Congressional Gold Medal in April 2008.
In his death, Dr. DeBakey was the first Houston resident given the honor of lying in state at City Hall and, at the request of his family, he lay dressed in his characteristic glasses, scrubs, and white coat for viewing by long lines of the general public.n
Sources and suggested readingsHeart Fail. Clin. 2007;3:117-20.J. Vasc. Surg. 1996;23:1031-4.
Dr. Michael Ellis DeBakey, pioneer heart surgeon and medical device innovator, died July 11, 2008, in Houston, about 2 months shy of his 100th birthday on Sept. 7.
In his lifetime, Dr. DeBakey was renowned for his immense contributions to the progress of medical science, such that he was declared a "living legend" by the Library of Congress and was this year awarded a Congressional Gold Medal for his lifetime achievements, in particular his pioneering work as a heart surgeon.
And although it was as a heart surgeon that Dr. DeBakey gained greatest fame, his accomplisments in vascular surgery were unique and profound, from his development of the Dacron graft to his active membership in the SVS, and his role as founder and first editor of the Journal of Vascular Surgery.
Even before Dr. DeBakey received his medical degree from Tulane University in 1932, he began his contributions to modern medicine by developing a small continuous flow–roller pump designed to improve blood transfusion—a device that would later be used by Dr. John Gibbon as a crucial component of his heart-lung machine. And in 1939, with his mentor, Dr. Alton Ochsner, Dr. DeBakey suggested a strong link between smoking and lung cancer.
After internships in New Orleans and surgical training in Europe, Dr. DeBakey volunteered for service during World War II and was assigned to the U.S. Army Surgeon General’s office. From his observations in the field, he became convinced of the need for a mobile surgical unit that would give soldiers access to high-level medical treatment on the combat field and convinced the surgeon general to form what would become the mobile army surgical hospitals (MASH units)—an innovation that gained him the U.S. Army Legion of Merit in 1945.
His government service continued throughout his civilian career, as he helped to establish the Veterans Administration medical center research system. He also initiated the movement that in 1956 took the Army’s poorly housed medical library and used it to create the National Library of Medicine, of which he was first board member and then chairman. He served three terms on the National Heart, Lung, and Blood Advisory Council as well. He was responsible for helping establish health care systems in a host of countries, including Belgium, China, Egypt, England, Germany, Saudi Arabia, Australia, and numerous other Middle Eastern and Central and South American nations.
According to the Web site of Baylor College of Medicine’s department of surgery, where he spent almost his entire postwar career, Dr. DeBakey operated on more than 60,000 patients in the Houston area alone. But these were not all just standard operations. In 1953, he performed the first successful carotid endarterectomy, as well as the first successful removal and graft replacement of a fusiform thoracic aortic aneurysm, and in 1954, the first successful resection and graft replacement of an aneurysm of the distal aortic arch and upper descending thoracic aorta.
In 1955 he performed the first successful resection of a thoracoabdominal aortic aneurysm using the DeBakey Dacron graft—the first artificial arterial graft of its kind.
"If we now tried to develop the Dacron graft the way we developed it, I am not sure we would have it today with the way they regulate things. ...When I went down to the department store . . . they said, ‘We are fresh out of nylon, but we do have a new material called Dacron.’ I felt it, and it looked good to me. So I bought a yard of it. . . . I took this yard of Dacron cloth, I cut two sheets the width I wanted, sewed the edges on each side, and made a tube out of it. . . . We put the graft on a stent, wrapped nylon thread around it, pushed it together, and baked it. . . . After about two or three years of laboratory work on my own [including experiments in dogs], I decided that it was time to put the graft in a human being. I did not have a committee to approve it. . . . In 1954, I put the first one in during an abdominal aortic aneurysm. That first patient lived, I think, for 13 years and never had any trouble," Dr. DeBakey related in an interview published in 1996 in the Journal of Vascular Surgery.
And among his other pioneering surgical developments, in 1964, Dr. DeBakey was the first to perform a successful coronary artery bypass, using a portion of leg vein as the graft, in what is now one of the most commonly performed heart operations—coronary artery bypass grafting.
As if surgically repairing failing hearts was not enough, Dr. DeBakey became a pioneer of artificial heart research and of cardiac assist devices. On July 18, 1963, after years of animal research, he performed the first successful human implantation of a left ventricular assist device (LVAD), one which he devised; the patient died after 4 days from causes unrelated to the technology. In 1966, Dr. DeBakey’s redesigned, extracorporeal pneumatic pump was used in a 37-year-old woman who could not be weaned from the heart-lung machine after dual valve replacement. After 10 days of LVAD support, she recovered sufficiently for the pump to be removed and she survived. This pump served as the basis of Dr. DeBakey’s first total artificial heart model, created in 1968.
Dr. DeBakey was honored profusely throughout his lifetime by the medical community and the general public. Numerous medical facilities are named after him in this country and around the world. He received countless awards for his technical and social achievements in medicine. Among these honors were the American Medical Association’s Distinguished Service Award (1959), the Albert Lasker Award for Clinical Medical Research (1963), the Presidential Medal of Freedom (1969), and the National Medal of Science (1987). More recently, he was the first foreign member elected to the Russian Academy of Sciences (1999), was given the Library of Congress Bicentennial Living Legend Award (2000), and was awarded the Congressional Gold Medal in April 2008.
In his death, Dr. DeBakey was the first Houston resident given the honor of lying in state at City Hall and, at the request of his family, he lay dressed in his characteristic glasses, scrubs, and white coat for viewing by long lines of the general public.n
Sources and suggested readingsHeart Fail. Clin. 2007;3:117-20.J. Vasc. Surg. 1996;23:1031-4.
Duty-Hour Surveys Separate Interns, Program Directors
Interns beginning their surgical training under the new resident duty-hour standards appear to be less pessimistic than program directors, but they still show significant concern that these new regulations will have a detrimental effect on the quality of their training, according to the results of separate surveys of surgical interns in general surgery residency programs and national surgical program directors.
The Accreditation Council for Graduate Medical Education (ACGME) implemented the new standards in July 2011 to include increased supervision and a 16-hour shift maximum for postgraduate year 1 residents, according to a report published in the June issue of Archives of Surgery.
In the summer of 2011, the researchers surveyed all 215 surgical interns in 11 general surgical residency programs distributed across the country to assess their perceptions of how the new duty-hour requirements would affect continuity of care, resident fatigue, and development in the six core ACGME competencies, according to Dr. Ryan M. Antiel of the Mayo Clinic, Rochester, Minn., and his colleagues. Perceptions were measured using a 3-point scale (increase, decrease, no change) for each item. A total of 179 (83.3%) completed the survey. Most respondents (68.7%) were men and were younger than 29 years of age (73%), with 102 categorical interns (57%) and 76 preliminary interns (42.5%), and 1 nonrespondent to this question (Arch. Surg. 2012;147:536-41).
Results of the resident survey were compared with those of an earlier survey of 134 program directors conducted by Dr. Antiel and his colleagues (Mayo Clin. Proc. 2011;86:185-91).
The great majority of the interns (80.3%) indicated that the new restrictions would decrease their ability to achieve continuity with hospitalized patients, and more than half (57.6%) stated that there would be a decrease in the coordination of patient care. Slightly fewer than half (48%) believed it would interfere with their acquisition of new medical knowledge.
"Most of the surgical interns (67.4%) believed that the duty-hour restrictions will decrease their time spent in the operating room," the researchers added. They also indicated that the new standards would decrease their development of surgical skills (52.8%); their time spent with patients on the floor (51.1%); and their overall educational experience (51.1%). Categorical interns were significantly more likely to believe that the changes would decrease both quality and safety of patient care (odds ratio, 2.6).
However, some optimism was also expressed: In all, 61.5% of interns believed that the new standards would decrease resident fatigue; 66.5% indicated that the new hours would increase or not change quality and safety of patient care; 72.1% indicated the same for the ability to effectively communicate with patients, families, and other health professionals; 74.7% indicated the same for the resident’s investigation and self-evaluation of their own patient care; and 70.2% felt that the impact would be neutral or favorable in the area of responsiveness to patient needs that supersede self-interest.
Compared with the program directors surveyed, a significantly higher proportion of interns believed that the new changes would improve or not change residents’ performance. And a significantly larger percentage of program directors agreed that the new changes would decrease coordination of patient care and residents’ acquisition of medical knowledge (76.9% vs. 48.0%). Perhaps most notably in terms of cross-perceptions, most interns (61.5%) believed that the new changes would decrease fatigue, whereas 85.1% of program directors believed that the new hours would increase fatigue, presumably by increasing the intensity of effort and accomplishments required in that shorter amount of time, according to the authors.
The researchers pointed out several limitations to their study beyond those intrinsic to surveys. Attitudes of residents may change over time, although the survey was most concerned with the perception of incoming interns. Also, program directors were not chosen randomly, and some regions may have been underrepresented. In addition, attitudes cannot be taken as evidence of the actual results of duty-hour restrictions on training, only the perceptions of that effect. But in the absence of defined metrics for assessing the effect of duty-hour restrictions on training, the attitudes of those most involved in training may be the best metric available, they noted.
"As residency programs attempt to adapt to the new regulations, surgical interns have significant concerns about the implications of these regulations on their training. The opinions of these interns, although markedly more optimistic than those of surgical program directors, reflect a persistent concern within the surgical community regarding the effects of work-hour restrictions on surgical training," they concluded.
The authors had no disclosures.
Eliminating two important limitations of this study might have put the interns more "in sync" with the program directors. First, large university programs constituted 10 of the 11 surveyed, and I suspect that those residents would be less concerned about duty-hour restrictions (because more of them subsequently choose fellowships and are less likely to go straight into general surgery practice) than would those from nonuniversity or community programs. Second, for 42.5% of the interns surveyed, there was no distinction made between those hoping to go into general surgery vs. those on track for surgical subspecialties, who are less likely to be concerned for the same reason of expecting additional training.
Even when we ignore the limitations of this study, I believe it shows that the "line in the sand" for the entire surgical community – residents and attendings – is no further resident duty-hour restrictions.
Mark L. Friedell, M.D., is from the department of surgery at the University of Missouri–Kansas City. His remarks are abstracted from an invited critique that accompanied the article (Arch. Surg. 2012;147:541). He reported having no disclosures.
Eliminating two important limitations of this study might have put the interns more "in sync" with the program directors. First, large university programs constituted 10 of the 11 surveyed, and I suspect that those residents would be less concerned about duty-hour restrictions (because more of them subsequently choose fellowships and are less likely to go straight into general surgery practice) than would those from nonuniversity or community programs. Second, for 42.5% of the interns surveyed, there was no distinction made between those hoping to go into general surgery vs. those on track for surgical subspecialties, who are less likely to be concerned for the same reason of expecting additional training.
Even when we ignore the limitations of this study, I believe it shows that the "line in the sand" for the entire surgical community – residents and attendings – is no further resident duty-hour restrictions.
Mark L. Friedell, M.D., is from the department of surgery at the University of Missouri–Kansas City. His remarks are abstracted from an invited critique that accompanied the article (Arch. Surg. 2012;147:541). He reported having no disclosures.
Eliminating two important limitations of this study might have put the interns more "in sync" with the program directors. First, large university programs constituted 10 of the 11 surveyed, and I suspect that those residents would be less concerned about duty-hour restrictions (because more of them subsequently choose fellowships and are less likely to go straight into general surgery practice) than would those from nonuniversity or community programs. Second, for 42.5% of the interns surveyed, there was no distinction made between those hoping to go into general surgery vs. those on track for surgical subspecialties, who are less likely to be concerned for the same reason of expecting additional training.
Even when we ignore the limitations of this study, I believe it shows that the "line in the sand" for the entire surgical community – residents and attendings – is no further resident duty-hour restrictions.
Mark L. Friedell, M.D., is from the department of surgery at the University of Missouri–Kansas City. His remarks are abstracted from an invited critique that accompanied the article (Arch. Surg. 2012;147:541). He reported having no disclosures.
Interns beginning their surgical training under the new resident duty-hour standards appear to be less pessimistic than program directors, but they still show significant concern that these new regulations will have a detrimental effect on the quality of their training, according to the results of separate surveys of surgical interns in general surgery residency programs and national surgical program directors.
The Accreditation Council for Graduate Medical Education (ACGME) implemented the new standards in July 2011 to include increased supervision and a 16-hour shift maximum for postgraduate year 1 residents, according to a report published in the June issue of Archives of Surgery.
In the summer of 2011, the researchers surveyed all 215 surgical interns in 11 general surgical residency programs distributed across the country to assess their perceptions of how the new duty-hour requirements would affect continuity of care, resident fatigue, and development in the six core ACGME competencies, according to Dr. Ryan M. Antiel of the Mayo Clinic, Rochester, Minn., and his colleagues. Perceptions were measured using a 3-point scale (increase, decrease, no change) for each item. A total of 179 (83.3%) completed the survey. Most respondents (68.7%) were men and were younger than 29 years of age (73%), with 102 categorical interns (57%) and 76 preliminary interns (42.5%), and 1 nonrespondent to this question (Arch. Surg. 2012;147:536-41).
Results of the resident survey were compared with those of an earlier survey of 134 program directors conducted by Dr. Antiel and his colleagues (Mayo Clin. Proc. 2011;86:185-91).
The great majority of the interns (80.3%) indicated that the new restrictions would decrease their ability to achieve continuity with hospitalized patients, and more than half (57.6%) stated that there would be a decrease in the coordination of patient care. Slightly fewer than half (48%) believed it would interfere with their acquisition of new medical knowledge.
"Most of the surgical interns (67.4%) believed that the duty-hour restrictions will decrease their time spent in the operating room," the researchers added. They also indicated that the new standards would decrease their development of surgical skills (52.8%); their time spent with patients on the floor (51.1%); and their overall educational experience (51.1%). Categorical interns were significantly more likely to believe that the changes would decrease both quality and safety of patient care (odds ratio, 2.6).
However, some optimism was also expressed: In all, 61.5% of interns believed that the new standards would decrease resident fatigue; 66.5% indicated that the new hours would increase or not change quality and safety of patient care; 72.1% indicated the same for the ability to effectively communicate with patients, families, and other health professionals; 74.7% indicated the same for the resident’s investigation and self-evaluation of their own patient care; and 70.2% felt that the impact would be neutral or favorable in the area of responsiveness to patient needs that supersede self-interest.
Compared with the program directors surveyed, a significantly higher proportion of interns believed that the new changes would improve or not change residents’ performance. And a significantly larger percentage of program directors agreed that the new changes would decrease coordination of patient care and residents’ acquisition of medical knowledge (76.9% vs. 48.0%). Perhaps most notably in terms of cross-perceptions, most interns (61.5%) believed that the new changes would decrease fatigue, whereas 85.1% of program directors believed that the new hours would increase fatigue, presumably by increasing the intensity of effort and accomplishments required in that shorter amount of time, according to the authors.
The researchers pointed out several limitations to their study beyond those intrinsic to surveys. Attitudes of residents may change over time, although the survey was most concerned with the perception of incoming interns. Also, program directors were not chosen randomly, and some regions may have been underrepresented. In addition, attitudes cannot be taken as evidence of the actual results of duty-hour restrictions on training, only the perceptions of that effect. But in the absence of defined metrics for assessing the effect of duty-hour restrictions on training, the attitudes of those most involved in training may be the best metric available, they noted.
"As residency programs attempt to adapt to the new regulations, surgical interns have significant concerns about the implications of these regulations on their training. The opinions of these interns, although markedly more optimistic than those of surgical program directors, reflect a persistent concern within the surgical community regarding the effects of work-hour restrictions on surgical training," they concluded.
The authors had no disclosures.
Interns beginning their surgical training under the new resident duty-hour standards appear to be less pessimistic than program directors, but they still show significant concern that these new regulations will have a detrimental effect on the quality of their training, according to the results of separate surveys of surgical interns in general surgery residency programs and national surgical program directors.
The Accreditation Council for Graduate Medical Education (ACGME) implemented the new standards in July 2011 to include increased supervision and a 16-hour shift maximum for postgraduate year 1 residents, according to a report published in the June issue of Archives of Surgery.
In the summer of 2011, the researchers surveyed all 215 surgical interns in 11 general surgical residency programs distributed across the country to assess their perceptions of how the new duty-hour requirements would affect continuity of care, resident fatigue, and development in the six core ACGME competencies, according to Dr. Ryan M. Antiel of the Mayo Clinic, Rochester, Minn., and his colleagues. Perceptions were measured using a 3-point scale (increase, decrease, no change) for each item. A total of 179 (83.3%) completed the survey. Most respondents (68.7%) were men and were younger than 29 years of age (73%), with 102 categorical interns (57%) and 76 preliminary interns (42.5%), and 1 nonrespondent to this question (Arch. Surg. 2012;147:536-41).
Results of the resident survey were compared with those of an earlier survey of 134 program directors conducted by Dr. Antiel and his colleagues (Mayo Clin. Proc. 2011;86:185-91).
The great majority of the interns (80.3%) indicated that the new restrictions would decrease their ability to achieve continuity with hospitalized patients, and more than half (57.6%) stated that there would be a decrease in the coordination of patient care. Slightly fewer than half (48%) believed it would interfere with their acquisition of new medical knowledge.
"Most of the surgical interns (67.4%) believed that the duty-hour restrictions will decrease their time spent in the operating room," the researchers added. They also indicated that the new standards would decrease their development of surgical skills (52.8%); their time spent with patients on the floor (51.1%); and their overall educational experience (51.1%). Categorical interns were significantly more likely to believe that the changes would decrease both quality and safety of patient care (odds ratio, 2.6).
However, some optimism was also expressed: In all, 61.5% of interns believed that the new standards would decrease resident fatigue; 66.5% indicated that the new hours would increase or not change quality and safety of patient care; 72.1% indicated the same for the ability to effectively communicate with patients, families, and other health professionals; 74.7% indicated the same for the resident’s investigation and self-evaluation of their own patient care; and 70.2% felt that the impact would be neutral or favorable in the area of responsiveness to patient needs that supersede self-interest.
Compared with the program directors surveyed, a significantly higher proportion of interns believed that the new changes would improve or not change residents’ performance. And a significantly larger percentage of program directors agreed that the new changes would decrease coordination of patient care and residents’ acquisition of medical knowledge (76.9% vs. 48.0%). Perhaps most notably in terms of cross-perceptions, most interns (61.5%) believed that the new changes would decrease fatigue, whereas 85.1% of program directors believed that the new hours would increase fatigue, presumably by increasing the intensity of effort and accomplishments required in that shorter amount of time, according to the authors.
The researchers pointed out several limitations to their study beyond those intrinsic to surveys. Attitudes of residents may change over time, although the survey was most concerned with the perception of incoming interns. Also, program directors were not chosen randomly, and some regions may have been underrepresented. In addition, attitudes cannot be taken as evidence of the actual results of duty-hour restrictions on training, only the perceptions of that effect. But in the absence of defined metrics for assessing the effect of duty-hour restrictions on training, the attitudes of those most involved in training may be the best metric available, they noted.
"As residency programs attempt to adapt to the new regulations, surgical interns have significant concerns about the implications of these regulations on their training. The opinions of these interns, although markedly more optimistic than those of surgical program directors, reflect a persistent concern within the surgical community regarding the effects of work-hour restrictions on surgical training," they concluded.
The authors had no disclosures.
Major Finding: The majority of interns (80.3%) thought the new restrictions would decrease their ability to achieved continuity with hospitalized patients and that there would be a decrease in the coordination of patient care (57.6%). Fewer than half (48%) believed it would interfere with their acquisition of new medical knowledge.
Data Source: Researchers analyzed the results of a survey of 215 surgical interns in general surgery residency programs and compared them with those of an earlier survey of 134 national surgical program directors.
Disclosures: The authors reported they had no financial disclosures.
Surgical Mentors: Confronting Diversity and Generational Change
Surgical mentoring – be it at the level of students, residents, junior faculty, or staff – is at a crossroads, and will require growth and transformation in order to avert a crisis, according to expert analysis presented at the annual meeting of the Society for Vascular Surgery.
The workforce as a whole, and medical practice in its wake, are rapidly moving from a generation of baby boomers who have a relatively homogenous worldview with respect to work, family, and society, to a diverse and fundamentally different group comprising generations X, Y, and Z for whom those values seem quaint, unengaging, or simply wrong.
Does this chasm make the experiences of the older generation moot in the eyes of the younger? Does it undercut the likelihood of successful mentoring? Or can mentors adapt to the needs of the newer generations?
It would not matter so much if surgical mentoring weren’t such a critical part of professional development. But surgeons are trained through an apprentice system whereby technical expertise is passed down from one generation to the next, along with the mores and expectations of the particular group culture.
Surgery requires not only profound functional skill, but astute professional judgment. In principle, those with many years of practical experience are the ideal mentors for the neophyte. But complex lifestyle and demographic issues (such as new resident work-hour restrictions, career paths, governmental and societal mandates, and shifting worldviews) are emerging that will create an experiential gap and alter the previous mentor-mentee relationship.
Generational Crossroads
Sarah Sladek provided some perspective on the increasing diversity between mentors and those who will need to be mentored.
Ms. Sladek is the founder of XYZ University, a marketing and consulting company focused on generational change, especially in membership associations. She sees the passing of the torch from the baby boomers (or the "loyalty generation") to the XYZ generations as the greatest challenge surgeons will face in mentoring.
"Demographic shifts are threatening the stability of most of our industries ... because most of our industries, including yours, are dominated by the baby boomer generation. But just 3 years from now (in 2015), generation Y, the youngest generation in the workforce, will outnumber the baby boomer generation."
If a surgical practice does not currently reflect this shift, it will need to. However, the structural accommodations in workday/workweek hours, office hierarchies and protocols, benefits, and time off that were made to the boomer generation are not at all tailored to the widely differing expectations and attitudes of the younger XYZ generations.
She pointed out that even the 3,500 members of the Society for Vascular Surgery reflect the nature of this change: Some 800 of those members are retired, and although 150 new graduates annually become vascular surgeons, 160 are needed to fill available positions. Thus, it is important to determine how to hire, engage, and mentor the new generations.
An understanding and acceptance of these differences will lead to structural changes in the way businesses – including surgical practices, academic centers, and even hospitals – operate, according to Ms. Sladek.
One of the most obvious differences, she pointed out, is the lack of diversity among the baby boomers vs. the considerable diversity in race, ethnicity, culture, and expectations found in generations X, Y, and Z.
Baby boomers, she said, were raised to recapitulate the workforce and lifestyle patterns of the generations before them. In contrast, the new generations expect a level of flexibility, personal gratification, novelty, and personal and social fulfillment in life and work far beyond that of their parents’ generation. Some people blame the children’s television program "Mister Rogers’ Neighborhood," she said. "All of a sudden, we had a childhood educator telling children, ‘You are special. You are unique. Do what makes you happy.’ And so we saw this move from conformity to individuality, and we also saw a move toward incredible independence, [with their] being the first generation of latchkey children."
Furthermore, she said, the new generations lack a sense of job security: No longer can they expect to get a job after college and stay with the same organization throughout their career. And they are inundated with news, much of it bad, including the fall of politicians and corporations. They have been shaped by greater individuality, suspicion of authority, and a lot more rebellion. The evolution from an era of relatively hands-off parenting to one of extremely hands-on parenting has created XYZ generations with vastly different experiences, sets of expectations, and worldviews, compared with those of their boomer bosses.
And neither the workplace nor the educational system is yet designed to handle this change. Yet change they must, she said, or they will lose out in competing for these workers and keeping them productive once they have them.
Dealing With Diversity
In theory, optimal mentoring should involve mentors’ sharing of knowledge and experience with the like-minded individuals who can be most receptive. In practice, there may be no choice but to accommodate a seeming mismatch between mentor and student. Today’s mentors – whether program directors, senior surgeons, senior faculty members, or even a senior residents – may need to step outside of their own comfort zone in life experiences, interests, and worldviews.
Perhaps a key indicator of the rapidly developing need to manage workforce change was the fact that the SVS mentorship session was sponsored jointly by the SVS Women’s Leadership Committee and the SVS Diversity and Inclusion Committee. Indeed, Dr. Julie Freischlag, surgeon-in-chief and chair of the department of surgery at Johns Hopkins University, Baltimore, who was one of the key speakers, represented a major facet of this shift: Next year, Dr. Freischlag will be the first female SVS president.
Dr. Freischlag pointed out that mentoring is, first and foremost, a relationship between two people, and not a set of teaching rules. A key aspect of mentoring, therefore, is trust. A coach is there just to get you through your job, she said, whereas a mentor will stick by you and continue to help even when you decide not to follow in the mentor’s footsteps.
A good mentor is one who has authentic guidelines; who promotes mature, self-governing work teams; and who walks the walk. "You need to have sound judgment, independent thinking, a tendency to think divergently, and a good sense of humor so that they want to come see you," she noted. The mentees "are not going to look like you. They may not be from the same background as you. They are going to have different aspirations."
She emphasized the importance of helping people to grow, to learn how to decide what is best for them, and to identify their real interests – not what the mentor would do in their position. "When you hear what they need, you need to have other people talk to them, pointing them to sources of information and other colleagues, all the while being supportive and enthusiastic about the choices made, even if they are not the ones that you yourself would have chosen."
To be in the top 10 among surgical mentors, she gave this advice:
• Let go of your expectations; look at their expectations, not yours.
• Put things into perspective for them and set a good example.
• Agree to disagree on certain things.
• Make light of being overwhelmed yourself, and do not belittle their feelings of being overwhelmed by their number of choices, activities, and so on.
As for identity, sex, race, specialty, family issues, or other areas upon which a mentor may not feel able to advise, she said that it is the mentor’s job to find appropriate additional mentors. "As you mentor people who are not like you, you may need to ask a lot more questions about how they are feeling or what they want, because you really don’t know. And saying to someone else that you ‘know what they are going through’ isn’t true, because you really don’t. So ‘tell me more what it’s like to be a student from Nigeria; tell me more what it’s like to be the only woman in this training program; tell me more what it’s like to be a single mother in this situation’ – those are the questions you need to use."
Dr. Fredrick P. Beavers of the Washington Hospital Center in Washington, D.C., spoke to his own experiences as an African American and to those of minorities in the past who did not have demographically similar mentors. He pointed to the success of these pioneers as evidence that mentorship does not require having identical sex, race, nationality, or other characteristics, but rather an ability to transcend such differences in terms of the values, respect, and true concern for the development and well-being of the mentored.
However, that is not to say that there is not a problem in the pipeline from medical school to the workforce. He cited statistics showing that the percentage of minorities drops dramatically at every step upward in the medical workforce. The percentage of minority individuals decreases from medical school to the level of junior faculty and staff, and further decreases from the junior to senior level and beyond. "The impact a positive mentoring relationship can have on a junior faculty member is immeasurable. The impact that a negative mentoring relationship can have has been measured, and is reflected in these statistics," he concluded.
Collectively, the speakers agreed that not only are true mentors capable of mentoring outside their demographics, but perhaps they also must be prepared to mentor outside of their interests, experience, and even their worldviews. This is a change in perspective that must happen if the surgical professions are to thrive.
None of the speakers had any disclosures relevant to their talk other than Ms. Sladek, who consulted on the issue of generational change.
Surgical mentoring – be it at the level of students, residents, junior faculty, or staff – is at a crossroads, and will require growth and transformation in order to avert a crisis, according to expert analysis presented at the annual meeting of the Society for Vascular Surgery.
The workforce as a whole, and medical practice in its wake, are rapidly moving from a generation of baby boomers who have a relatively homogenous worldview with respect to work, family, and society, to a diverse and fundamentally different group comprising generations X, Y, and Z for whom those values seem quaint, unengaging, or simply wrong.
Does this chasm make the experiences of the older generation moot in the eyes of the younger? Does it undercut the likelihood of successful mentoring? Or can mentors adapt to the needs of the newer generations?
It would not matter so much if surgical mentoring weren’t such a critical part of professional development. But surgeons are trained through an apprentice system whereby technical expertise is passed down from one generation to the next, along with the mores and expectations of the particular group culture.
Surgery requires not only profound functional skill, but astute professional judgment. In principle, those with many years of practical experience are the ideal mentors for the neophyte. But complex lifestyle and demographic issues (such as new resident work-hour restrictions, career paths, governmental and societal mandates, and shifting worldviews) are emerging that will create an experiential gap and alter the previous mentor-mentee relationship.
Generational Crossroads
Sarah Sladek provided some perspective on the increasing diversity between mentors and those who will need to be mentored.
Ms. Sladek is the founder of XYZ University, a marketing and consulting company focused on generational change, especially in membership associations. She sees the passing of the torch from the baby boomers (or the "loyalty generation") to the XYZ generations as the greatest challenge surgeons will face in mentoring.
"Demographic shifts are threatening the stability of most of our industries ... because most of our industries, including yours, are dominated by the baby boomer generation. But just 3 years from now (in 2015), generation Y, the youngest generation in the workforce, will outnumber the baby boomer generation."
If a surgical practice does not currently reflect this shift, it will need to. However, the structural accommodations in workday/workweek hours, office hierarchies and protocols, benefits, and time off that were made to the boomer generation are not at all tailored to the widely differing expectations and attitudes of the younger XYZ generations.
She pointed out that even the 3,500 members of the Society for Vascular Surgery reflect the nature of this change: Some 800 of those members are retired, and although 150 new graduates annually become vascular surgeons, 160 are needed to fill available positions. Thus, it is important to determine how to hire, engage, and mentor the new generations.
An understanding and acceptance of these differences will lead to structural changes in the way businesses – including surgical practices, academic centers, and even hospitals – operate, according to Ms. Sladek.
One of the most obvious differences, she pointed out, is the lack of diversity among the baby boomers vs. the considerable diversity in race, ethnicity, culture, and expectations found in generations X, Y, and Z.
Baby boomers, she said, were raised to recapitulate the workforce and lifestyle patterns of the generations before them. In contrast, the new generations expect a level of flexibility, personal gratification, novelty, and personal and social fulfillment in life and work far beyond that of their parents’ generation. Some people blame the children’s television program "Mister Rogers’ Neighborhood," she said. "All of a sudden, we had a childhood educator telling children, ‘You are special. You are unique. Do what makes you happy.’ And so we saw this move from conformity to individuality, and we also saw a move toward incredible independence, [with their] being the first generation of latchkey children."
Furthermore, she said, the new generations lack a sense of job security: No longer can they expect to get a job after college and stay with the same organization throughout their career. And they are inundated with news, much of it bad, including the fall of politicians and corporations. They have been shaped by greater individuality, suspicion of authority, and a lot more rebellion. The evolution from an era of relatively hands-off parenting to one of extremely hands-on parenting has created XYZ generations with vastly different experiences, sets of expectations, and worldviews, compared with those of their boomer bosses.
And neither the workplace nor the educational system is yet designed to handle this change. Yet change they must, she said, or they will lose out in competing for these workers and keeping them productive once they have them.
Dealing With Diversity
In theory, optimal mentoring should involve mentors’ sharing of knowledge and experience with the like-minded individuals who can be most receptive. In practice, there may be no choice but to accommodate a seeming mismatch between mentor and student. Today’s mentors – whether program directors, senior surgeons, senior faculty members, or even a senior residents – may need to step outside of their own comfort zone in life experiences, interests, and worldviews.
Perhaps a key indicator of the rapidly developing need to manage workforce change was the fact that the SVS mentorship session was sponsored jointly by the SVS Women’s Leadership Committee and the SVS Diversity and Inclusion Committee. Indeed, Dr. Julie Freischlag, surgeon-in-chief and chair of the department of surgery at Johns Hopkins University, Baltimore, who was one of the key speakers, represented a major facet of this shift: Next year, Dr. Freischlag will be the first female SVS president.
Dr. Freischlag pointed out that mentoring is, first and foremost, a relationship between two people, and not a set of teaching rules. A key aspect of mentoring, therefore, is trust. A coach is there just to get you through your job, she said, whereas a mentor will stick by you and continue to help even when you decide not to follow in the mentor’s footsteps.
A good mentor is one who has authentic guidelines; who promotes mature, self-governing work teams; and who walks the walk. "You need to have sound judgment, independent thinking, a tendency to think divergently, and a good sense of humor so that they want to come see you," she noted. The mentees "are not going to look like you. They may not be from the same background as you. They are going to have different aspirations."
She emphasized the importance of helping people to grow, to learn how to decide what is best for them, and to identify their real interests – not what the mentor would do in their position. "When you hear what they need, you need to have other people talk to them, pointing them to sources of information and other colleagues, all the while being supportive and enthusiastic about the choices made, even if they are not the ones that you yourself would have chosen."
To be in the top 10 among surgical mentors, she gave this advice:
• Let go of your expectations; look at their expectations, not yours.
• Put things into perspective for them and set a good example.
• Agree to disagree on certain things.
• Make light of being overwhelmed yourself, and do not belittle their feelings of being overwhelmed by their number of choices, activities, and so on.
As for identity, sex, race, specialty, family issues, or other areas upon which a mentor may not feel able to advise, she said that it is the mentor’s job to find appropriate additional mentors. "As you mentor people who are not like you, you may need to ask a lot more questions about how they are feeling or what they want, because you really don’t know. And saying to someone else that you ‘know what they are going through’ isn’t true, because you really don’t. So ‘tell me more what it’s like to be a student from Nigeria; tell me more what it’s like to be the only woman in this training program; tell me more what it’s like to be a single mother in this situation’ – those are the questions you need to use."
Dr. Fredrick P. Beavers of the Washington Hospital Center in Washington, D.C., spoke to his own experiences as an African American and to those of minorities in the past who did not have demographically similar mentors. He pointed to the success of these pioneers as evidence that mentorship does not require having identical sex, race, nationality, or other characteristics, but rather an ability to transcend such differences in terms of the values, respect, and true concern for the development and well-being of the mentored.
However, that is not to say that there is not a problem in the pipeline from medical school to the workforce. He cited statistics showing that the percentage of minorities drops dramatically at every step upward in the medical workforce. The percentage of minority individuals decreases from medical school to the level of junior faculty and staff, and further decreases from the junior to senior level and beyond. "The impact a positive mentoring relationship can have on a junior faculty member is immeasurable. The impact that a negative mentoring relationship can have has been measured, and is reflected in these statistics," he concluded.
Collectively, the speakers agreed that not only are true mentors capable of mentoring outside their demographics, but perhaps they also must be prepared to mentor outside of their interests, experience, and even their worldviews. This is a change in perspective that must happen if the surgical professions are to thrive.
None of the speakers had any disclosures relevant to their talk other than Ms. Sladek, who consulted on the issue of generational change.
Surgical mentoring – be it at the level of students, residents, junior faculty, or staff – is at a crossroads, and will require growth and transformation in order to avert a crisis, according to expert analysis presented at the annual meeting of the Society for Vascular Surgery.
The workforce as a whole, and medical practice in its wake, are rapidly moving from a generation of baby boomers who have a relatively homogenous worldview with respect to work, family, and society, to a diverse and fundamentally different group comprising generations X, Y, and Z for whom those values seem quaint, unengaging, or simply wrong.
Does this chasm make the experiences of the older generation moot in the eyes of the younger? Does it undercut the likelihood of successful mentoring? Or can mentors adapt to the needs of the newer generations?
It would not matter so much if surgical mentoring weren’t such a critical part of professional development. But surgeons are trained through an apprentice system whereby technical expertise is passed down from one generation to the next, along with the mores and expectations of the particular group culture.
Surgery requires not only profound functional skill, but astute professional judgment. In principle, those with many years of practical experience are the ideal mentors for the neophyte. But complex lifestyle and demographic issues (such as new resident work-hour restrictions, career paths, governmental and societal mandates, and shifting worldviews) are emerging that will create an experiential gap and alter the previous mentor-mentee relationship.
Generational Crossroads
Sarah Sladek provided some perspective on the increasing diversity between mentors and those who will need to be mentored.
Ms. Sladek is the founder of XYZ University, a marketing and consulting company focused on generational change, especially in membership associations. She sees the passing of the torch from the baby boomers (or the "loyalty generation") to the XYZ generations as the greatest challenge surgeons will face in mentoring.
"Demographic shifts are threatening the stability of most of our industries ... because most of our industries, including yours, are dominated by the baby boomer generation. But just 3 years from now (in 2015), generation Y, the youngest generation in the workforce, will outnumber the baby boomer generation."
If a surgical practice does not currently reflect this shift, it will need to. However, the structural accommodations in workday/workweek hours, office hierarchies and protocols, benefits, and time off that were made to the boomer generation are not at all tailored to the widely differing expectations and attitudes of the younger XYZ generations.
She pointed out that even the 3,500 members of the Society for Vascular Surgery reflect the nature of this change: Some 800 of those members are retired, and although 150 new graduates annually become vascular surgeons, 160 are needed to fill available positions. Thus, it is important to determine how to hire, engage, and mentor the new generations.
An understanding and acceptance of these differences will lead to structural changes in the way businesses – including surgical practices, academic centers, and even hospitals – operate, according to Ms. Sladek.
One of the most obvious differences, she pointed out, is the lack of diversity among the baby boomers vs. the considerable diversity in race, ethnicity, culture, and expectations found in generations X, Y, and Z.
Baby boomers, she said, were raised to recapitulate the workforce and lifestyle patterns of the generations before them. In contrast, the new generations expect a level of flexibility, personal gratification, novelty, and personal and social fulfillment in life and work far beyond that of their parents’ generation. Some people blame the children’s television program "Mister Rogers’ Neighborhood," she said. "All of a sudden, we had a childhood educator telling children, ‘You are special. You are unique. Do what makes you happy.’ And so we saw this move from conformity to individuality, and we also saw a move toward incredible independence, [with their] being the first generation of latchkey children."
Furthermore, she said, the new generations lack a sense of job security: No longer can they expect to get a job after college and stay with the same organization throughout their career. And they are inundated with news, much of it bad, including the fall of politicians and corporations. They have been shaped by greater individuality, suspicion of authority, and a lot more rebellion. The evolution from an era of relatively hands-off parenting to one of extremely hands-on parenting has created XYZ generations with vastly different experiences, sets of expectations, and worldviews, compared with those of their boomer bosses.
And neither the workplace nor the educational system is yet designed to handle this change. Yet change they must, she said, or they will lose out in competing for these workers and keeping them productive once they have them.
Dealing With Diversity
In theory, optimal mentoring should involve mentors’ sharing of knowledge and experience with the like-minded individuals who can be most receptive. In practice, there may be no choice but to accommodate a seeming mismatch between mentor and student. Today’s mentors – whether program directors, senior surgeons, senior faculty members, or even a senior residents – may need to step outside of their own comfort zone in life experiences, interests, and worldviews.
Perhaps a key indicator of the rapidly developing need to manage workforce change was the fact that the SVS mentorship session was sponsored jointly by the SVS Women’s Leadership Committee and the SVS Diversity and Inclusion Committee. Indeed, Dr. Julie Freischlag, surgeon-in-chief and chair of the department of surgery at Johns Hopkins University, Baltimore, who was one of the key speakers, represented a major facet of this shift: Next year, Dr. Freischlag will be the first female SVS president.
Dr. Freischlag pointed out that mentoring is, first and foremost, a relationship between two people, and not a set of teaching rules. A key aspect of mentoring, therefore, is trust. A coach is there just to get you through your job, she said, whereas a mentor will stick by you and continue to help even when you decide not to follow in the mentor’s footsteps.
A good mentor is one who has authentic guidelines; who promotes mature, self-governing work teams; and who walks the walk. "You need to have sound judgment, independent thinking, a tendency to think divergently, and a good sense of humor so that they want to come see you," she noted. The mentees "are not going to look like you. They may not be from the same background as you. They are going to have different aspirations."
She emphasized the importance of helping people to grow, to learn how to decide what is best for them, and to identify their real interests – not what the mentor would do in their position. "When you hear what they need, you need to have other people talk to them, pointing them to sources of information and other colleagues, all the while being supportive and enthusiastic about the choices made, even if they are not the ones that you yourself would have chosen."
To be in the top 10 among surgical mentors, she gave this advice:
• Let go of your expectations; look at their expectations, not yours.
• Put things into perspective for them and set a good example.
• Agree to disagree on certain things.
• Make light of being overwhelmed yourself, and do not belittle their feelings of being overwhelmed by their number of choices, activities, and so on.
As for identity, sex, race, specialty, family issues, or other areas upon which a mentor may not feel able to advise, she said that it is the mentor’s job to find appropriate additional mentors. "As you mentor people who are not like you, you may need to ask a lot more questions about how they are feeling or what they want, because you really don’t know. And saying to someone else that you ‘know what they are going through’ isn’t true, because you really don’t. So ‘tell me more what it’s like to be a student from Nigeria; tell me more what it’s like to be the only woman in this training program; tell me more what it’s like to be a single mother in this situation’ – those are the questions you need to use."
Dr. Fredrick P. Beavers of the Washington Hospital Center in Washington, D.C., spoke to his own experiences as an African American and to those of minorities in the past who did not have demographically similar mentors. He pointed to the success of these pioneers as evidence that mentorship does not require having identical sex, race, nationality, or other characteristics, but rather an ability to transcend such differences in terms of the values, respect, and true concern for the development and well-being of the mentored.
However, that is not to say that there is not a problem in the pipeline from medical school to the workforce. He cited statistics showing that the percentage of minorities drops dramatically at every step upward in the medical workforce. The percentage of minority individuals decreases from medical school to the level of junior faculty and staff, and further decreases from the junior to senior level and beyond. "The impact a positive mentoring relationship can have on a junior faculty member is immeasurable. The impact that a negative mentoring relationship can have has been measured, and is reflected in these statistics," he concluded.
Collectively, the speakers agreed that not only are true mentors capable of mentoring outside their demographics, but perhaps they also must be prepared to mentor outside of their interests, experience, and even their worldviews. This is a change in perspective that must happen if the surgical professions are to thrive.
None of the speakers had any disclosures relevant to their talk other than Ms. Sladek, who consulted on the issue of generational change.